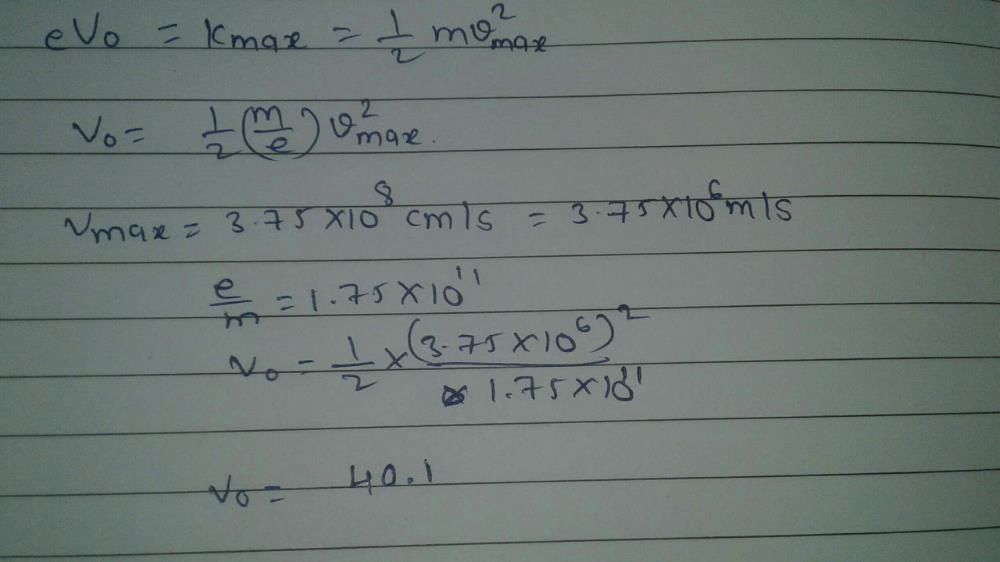All Exams >
NEET >
Physics Class 12 >
All Questions
All questions of Dual Nature of Radiation and Matter for NEET Exam
What is the de Broglie wavelength associated with ... morean electron, accelerated through a potential difference of 100 volts?a)12.3 nmb)0.127 nmc)123 nmd)1.23 nmCorrect answer is option 'B'. Can you explain this answer?
|
|
Geetika Shah answered |

and as we know 1Å = 0.1 nm, so 1.227Å = 0.122 nm
Number of ejected photoelectrons increases with increase- a)never
- b)in frequency of light
- c)in wavelength of light
- d)in intensity of light
Correct answer is option 'D'. Can you explain this answer?
Number of ejected photoelectrons increases with increase
a)
never
b)
in frequency of light
c)
in wavelength of light
d)
in intensity of light
|
|
Rohan Singh answered |
A photon is the smallest possible quantum of light. In general when you turn up the intensity of light you are increasing the number of photons per second that are emitted by the light source. Therefore the intensity of the light can indeed be changed independently of the frequency (or color) of the light.
In order to increase the kinetic energy of ejected photoelectrons, there should be an increase in- a)wavelength of radiation
- b)intensity of radiation
- c)frequency of radiation
- d)Both the wavelength and intensity of radiation
Correct answer is option 'C'. Can you explain this answer?
In order to increase the kinetic energy of ejected photoelectrons, there should be an increase in
a)
wavelength of radiation
b)
intensity of radiation
c)
frequency of radiation
d)
Both the wavelength and intensity of radiation
|
|
Arjun Singhania answered |
The kinetic energy of emitted photoelectrons should increase with the light amplitude. The rate of electron emission, which is proportional to the measured electric current, should increase as the light frequency is increased.
The energy of the incident photon is 20 eV and the work function of the photosensitive metal is 10 eV. What is the stopping potential?- a)30 V
- b)5 V
- c)10 V
- d)15 V
Correct answer is option 'C'. Can you explain this answer?
The energy of the incident photon is 20 eV and the work function of the photosensitive metal is 10 eV. What is the stopping potential?
a)
30 V
b)
5 V
c)
10 V
d)
15 V
|
|
Krishna Iyer answered |
Stopping potential (Vo) is given by
Vo=W/q where W is the work function and q are the charge of an electron.
Given W=20eV−10eV=10eV. Also, q=e
Hence, Vo=(10eV)/e=10V
Vo=W/q where W is the work function and q are the charge of an electron.
Given W=20eV−10eV=10eV. Also, q=e
Hence, Vo=(10eV)/e=10V
Photons of energy 6 eV are incident on a potassium surface of a work function 2.1 eV. What is the stopping potential?- a)-3.9V
- b)-8.1V
- c)-5V
- d)-1.9V
Correct answer is 'A'. Can you explain this answer?
Photons of energy 6 eV are incident on a potassium surface of a work function 2.1 eV. What is the stopping potential?
a)
-3.9V
b)
-8.1V
c)
-5V
d)
-1.9V
|
|
Priyanka Sharma answered |
From photo-electric equation, eV0= E−φ
eV0=(6−2.1)eV
V0= 3.9 V
stopping potential is a negative potential to stop e- at saturated current .
eV0=(6−2.1)eV
V0= 3.9 V
stopping potential is a negative potential to stop e- at saturated current .
Light of frequency 1.5 times the threshold frequency is incident on a photosensitive material .If the frequency is halved and the intensity is doubled, the photoelectric current becomes- a)Doubled
- b)Quadrupled
- c)Halved
- d)zero
Correct answer is option 'D'. Can you explain this answer?
Light of frequency 1.5 times the threshold frequency is incident on a photosensitive material .If the frequency is halved and the intensity is doubled, the photoelectric current becomes
a)
Doubled
b)
Quadrupled
c)
Halved
d)
zero

|
Ayush Joshi answered |
If the frequency is halved and intensity is doubled, the frequency of incident light will become 15/2 = 0.75 times the threshold frequency. So, as ν<νo Hence, photoelectric current will be zero.
The work function of a photoelectric material is 3.32 eV. The threshold frequency will be equal to- a)8 ×1014 HZ
- b)9 ×1014 HZ
- c)7 ×1014 HZ
- d)6 ×1014 HZ
Correct answer is option 'A'. Can you explain this answer?
The work function of a photoelectric material is 3.32 eV. The threshold frequency will be equal to
a)
8 ×1014 HZ
b)
9 ×1014 HZ
c)
7 ×1014 HZ
d)
6 ×1014 HZ
|
|
Anu Sharma answered |
The threshold frequency can be calculated using the formula:
threshold frequency = work function / Planck's constant
Given that the work function is 3.32 eV, we need to convert it to joules by multiplying it by the conversion factor 1.602 x 10^-19 J/eV:
work function = 3.32 eV * 1.602 x 10^-19 J/eV = 5.31264 x 10^-19 J
The value of Planck's constant is 6.626 x 10^-34 J·s.
Therefore, the threshold frequency is:
threshold frequency = 5.31264 x 10^-19 J / (6.626 x 10^-34 J·s)
threshold frequency ≈ 8.03 x 10^14 Hz
So, the threshold frequency will be approximately 8.03 x 10^14 Hz.
threshold frequency = work function / Planck's constant
Given that the work function is 3.32 eV, we need to convert it to joules by multiplying it by the conversion factor 1.602 x 10^-19 J/eV:
work function = 3.32 eV * 1.602 x 10^-19 J/eV = 5.31264 x 10^-19 J
The value of Planck's constant is 6.626 x 10^-34 J·s.
Therefore, the threshold frequency is:
threshold frequency = 5.31264 x 10^-19 J / (6.626 x 10^-34 J·s)
threshold frequency ≈ 8.03 x 10^14 Hz
So, the threshold frequency will be approximately 8.03 x 10^14 Hz.
In a photon-particle collision (such as photon-electron collision) the quantity which is not conserved is- a)total momentum
- b)number of photons
- c)total energy
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
In a photon-particle collision (such as photon-electron collision) the quantity which is not conserved is
a)
total momentum
b)
number of photons
c)
total energy
d)
None of the above
|
|
Rohan Singh answered |
Energy and momentum are conserved, resulting in a reduction of both for the scattered photon. ... This phenomenon could be handled as a collision between two particles—a photon and an electron at rest in the material. Energy and momentum are conserved in the collision.
A photo-sensitive material would emit electrons, if excited by photons beyond a threshold. To overcome the threshold, one would increase the:- a) voltage applied to the light source
- b) intensity of light
- c) wavelength of light
- d) frequency of light
Correct answer is option 'D'. Can you explain this answer?
A photo-sensitive material would emit electrons, if excited by photons beyond a threshold. To overcome the threshold, one would increase the:
a)
voltage applied to the light source
b)
intensity of light
c)
wavelength of light
d)
frequency of light
|
|
Rohan Singh answered |
The emission of photoelectron takes place only, when the frequency of the incident light is above a certain critical value, characteristic of that metal. The critical value of frequency is known as the threshold frequency for the metal of the emitting electrode.
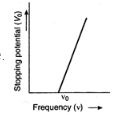
Suppose that when light of certain frequency is incident over a metal surface, the photo- electrons are emitted. To take photoelectric current zero, a particular value of stopping potential will be needed. If we go on reducing the frequency of incident light, the value of stopping potential will also go on decreasing. At certain value of frequency v0, the photoelectric current will become zero, even when no retarding potential is applied. This frequency vq corresponds to the threshold for the metal surface. The emission of photoelectrons does not take place, till frequency of incident light is below this value.

Can you explain the answer of this question below:Light from a bulb is falling on a wooden table but no photo electrons are emitted as
- A:
Work function of wood is less
- B:
Work function of wood is more
- C:
It depends on the frequency
- D:
It is independent of work function
The answer is b.
Light from a bulb is falling on a wooden table but no photo electrons are emitted as
Work function of wood is less
Work function of wood is more
It depends on the frequency
It is independent of work function
|
|
Rishika Patel answered |
Explanation:
When light falls on a metal surface, electrons may be emitted from the metal surface. This phenomenon is known as the photoelectric effect. The electrons emitted from the metal surface are called photoelectrons.
The photoelectric effect can be explained by considering that light is made up of photons. Each photon has a certain amount of energy, given by its frequency. When a photon strikes a metal surface, its energy can be transferred to an electron in the metal. If the energy of the photon is greater than the work function of the metal, the electron can be emitted from the metal surface.
In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it. Therefore, no photoelectrons are emitted. This is because the energy of the photons is not enough to overcome the work function of the wood.
Key Points:
- The photoelectric effect is the emission of electrons from a metal surface when light falls on it.
- The energy of a photon is given by its frequency.
- If the energy of a photon is greater than the work function of the metal, electrons can be emitted from the metal surface.
- In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it, so no photoelectrons are emitted.
When light falls on a metal surface, electrons may be emitted from the metal surface. This phenomenon is known as the photoelectric effect. The electrons emitted from the metal surface are called photoelectrons.
The photoelectric effect can be explained by considering that light is made up of photons. Each photon has a certain amount of energy, given by its frequency. When a photon strikes a metal surface, its energy can be transferred to an electron in the metal. If the energy of the photon is greater than the work function of the metal, the electron can be emitted from the metal surface.
In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it. Therefore, no photoelectrons are emitted. This is because the energy of the photons is not enough to overcome the work function of the wood.
Key Points:
- The photoelectric effect is the emission of electrons from a metal surface when light falls on it.
- The energy of a photon is given by its frequency.
- If the energy of a photon is greater than the work function of the metal, electrons can be emitted from the metal surface.
- In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it, so no photoelectrons are emitted.
Sodium surface is illuminated by ultraviolet and visible radiation successively and the stopping potential determined. The stopping potential is- a)Greater with visible light
- b)Equal in both cases
- c)Greater with ultraviolet light
- d)Infinite in both cases
Correct answer is 'C'. Can you explain this answer?
Sodium surface is illuminated by ultraviolet and visible radiation successively and the stopping potential determined. The stopping potential is
a)
Greater with visible light
b)
Equal in both cases
c)
Greater with ultraviolet light
d)
Infinite in both cases
|
|
Riya Banerjee answered |
λ for U.V is less than λ for visible light
ν for U.V is greater than ν for visible light
∴ potential is greater for U.V light.
as K.Eα1/λ
ν for U.V is greater than ν for visible light
∴ potential is greater for U.V light.
as K.Eα1/λ
An α – particle and a deutron are accelerated through the same potential difference. What will be the ratio of their de-Broglie wavelength?- a)1 / 3
- b)1 / 5
- c)1 / 4
- d)1 / 2
Correct answer is 'D'. Can you explain this answer?
An α – particle and a deutron are accelerated through the same potential difference. What will be the ratio of their de-Broglie wavelength?
a)
1 / 3
b)
1 / 5
c)
1 / 4
d)
1 / 2
|
|
Abhay Iyer answered |
Mass of alpha(a)= 4.
mass of deutron(d) =2.
wavelength=h/mv
Potential are same of both particle so their speed will be same .
Here, wavelength is inversely proportional to mass
so,
wavelength of alpha/wavelength of deutron = mass of deutron /mass of alpha.= 2/4=1/2
mass of deutron(d) =2.
wavelength=h/mv
Potential are same of both particle so their speed will be same .
Here, wavelength is inversely proportional to mass
so,
wavelength of alpha/wavelength of deutron = mass of deutron /mass of alpha.= 2/4=1/2
The de-Broglie wavelength of an electron is 1.0 nm. What is the retarding potential required to stop it?- a)1.5 V
- b)3.5 V
- c)6.5 V
- d)4.5 V
Correct answer is option 'A'. Can you explain this answer?
The de-Broglie wavelength of an electron is 1.0 nm. What is the retarding potential required to stop it?
a)
1.5 V
b)
3.5 V
c)
6.5 V
d)
4.5 V
|
|
Neha Sharma answered |
λ=h/P
Here P= √2mk
K=kinetic energy
λ=h/√2mk
By energy conversion
K=eVs
λ=h/√2meVs
√Vs=h/ λ√2me
Vs=h2/ λ2me
Vs=(6.626x10-34)2/(1x10-9)2x2x9.11x10-31xe
Where e=1.602x10-19
Vs=43.9x10-68/29.1x10-68
=1.508v
Vs=1.5v
Here P= √2mk
K=kinetic energy
λ=h/√2mk
By energy conversion
K=eVs
λ=h/√2meVs
√Vs=h/ λ√2me
Vs=h2/ λ2me
Vs=(6.626x10-34)2/(1x10-9)2x2x9.11x10-31xe
Where e=1.602x10-19
Vs=43.9x10-68/29.1x10-68
=1.508v
Vs=1.5v
In an experiment of photoelectric emission for incident light of 4000 A0, the stopping potential is 2V. If the wavelength of incident light is made 3000 A0, then stopping potential will be- a)zero
- b)more than 2 volt
- c)2 Volt
- d)less than 2 volt
Correct answer is option 'B'. Can you explain this answer?
In an experiment of photoelectric emission for incident light of 4000 A0, the stopping potential is 2V. If the wavelength of incident light is made 3000 A0, then stopping potential will be
a)
zero
b)
more than 2 volt
c)
2 Volt
d)
less than 2 volt
|
|
Geetika Shah answered |
The maximum kinetic energy for the photoelectrons is
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
If Vo is the stopping potential then
eV0=h(c/λ)−ϕ .....................(since, ν=c/λ)
As per the problem, for incident light of 4000Ao, the stopping potential is 2V. When the wavelength of incident light is reduced to 3000Ao, then the stopping potential will increase to value more than 2V(as per the above equation).
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
If Vo is the stopping potential then
eV0=h(c/λ)−ϕ .....................(since, ν=c/λ)
As per the problem, for incident light of 4000Ao, the stopping potential is 2V. When the wavelength of incident light is reduced to 3000Ao, then the stopping potential will increase to value more than 2V(as per the above equation).
Light of wavelength 4000 Â is incident on a metal of work function 3.2 x 10-19 J. What is the maximum kinetic energy of the emitted electron?
- a)2.2 x 10-19 J
- b)1.75 x 10-19 J
- c)0.75 x 10-19 J
- d)1.1 x 10-19 J
Correct answer is option 'B'. Can you explain this answer?
Light of wavelength 4000 Â is incident on a metal of work function 3.2 x 10-19 J. What is the maximum kinetic energy of the emitted electron?
a)
2.2 x 10-19 J
b)
1.75 x 10-19 J
c)
0.75 x 10-19 J
d)
1.1 x 10-19 J
|
|
Om Desai answered |
Given,
λ= 4000 Å ;W=3.2× 10-19 j
as we know that
K.E = (hc/ λ) - W
= (6.6× 10-34 × 3 × 108/ 4000×10-10 )- 3.20× 10-19
= 1.75 × 10-19
λ= 4000 Å ;W=3.2× 10-19 j
as we know that
K.E = (hc/ λ) - W
= (6.6× 10-34 × 3 × 108/ 4000×10-10 )- 3.20× 10-19
= 1.75 × 10-19
Wavelength of light incident on a photo cell is 3000 Â, if stopping potential is 2.5 volts, then work function of the cathode of photo cell is- a)1.64 eV
- b)1.56 eV
- c)1.52 eV
- d)1.41 eV
Correct answer is option 'A'. Can you explain this answer?
Wavelength of light incident on a photo cell is 3000 Â, if stopping potential is 2.5 volts, then work function of the cathode of photo cell is
a)
1.64 eV
b)
1.56 eV
c)
1.52 eV
d)
1.41 eV
|
|
Neha Sharma answered |
The Stopping potential =2.5V.
or, Kinetic energy=2.5eV.
We know that,
Incident energy =work function + Kinetic energy.
To get incident energy in e.V,
We also know that,
The Incident energy =12400/λ Å
Incident energy=work function + kinetic energy.
12400/3000 = work function + 2.5e.v.
4.13-2.5 = work function
work function=1.64 e.V
or, Kinetic energy=2.5eV.
We know that,
Incident energy =work function + Kinetic energy.
To get incident energy in e.V,
We also know that,
The Incident energy =12400/λ Å
Incident energy=work function + kinetic energy.
12400/3000 = work function + 2.5e.v.
4.13-2.5 = work function
work function=1.64 e.V
In which case is electron emission from a metal not known?- a)Illuminating a metal surface with suitable light
- b)Applying a very strong magnetic field to a metal
- c)Applying a very strong electric field to a metal
- d)Heating the metal sufficiently
Correct answer is option 'B'. Can you explain this answer?
In which case is electron emission from a metal not known?
a)
Illuminating a metal surface with suitable light
b)
Applying a very strong magnetic field to a metal
c)
Applying a very strong electric field to a metal
d)
Heating the metal sufficiently

|
Divey Sethi answered |
The correct answer would be option B. Applying a very strong magnetic field.
Applying a very strong magnetic field to a metal we don’t know the electron emission.
Applying a very strong magnetic field to a metal we don’t know the electron emission.
Given h = 6.6 ×10−34 joule sec, the momentum of each photon in a given radiation is 3.3 ×10−29 kg metre/sec. The frequency of radiation is- a)1.6 ×1013Hz
- b)1.5 ×1013Hz
- c)1.7 ×1013Hz
- d)1.8 ×1013Hz
Correct answer is option 'B'. Can you explain this answer?
Given h = 6.6 ×10−34 joule sec, the momentum of each photon in a given radiation is 3.3 ×10−29 kg metre/sec. The frequency of radiation is
a)
1.6 ×1013Hz
b)
1.5 ×1013Hz
c)
1.7 ×1013Hz
d)
1.8 ×1013Hz
|
|
Lavanya Menon answered |
Frequency=C/ λ
λ=h/P
frequency=C P/ h
f=(3×108×3.3×10-29)/6.6×10-34
f=3× 1013/2
f=1.5×1013 Hz
λ=h/P
frequency=C P/ h
f=(3×108×3.3×10-29)/6.6×10-34
f=3× 1013/2
f=1.5×1013 Hz
Of the following moving with the same momentum, the one which has the largest wavelength is- a)a proton
- b)α -particle
- c)an electron
- d)all have the same de Broglie wavelength
Correct answer is option 'D'. Can you explain this answer?
Of the following moving with the same momentum, the one which has the largest wavelength is
a)
a proton
b)
α -particle
c)
an electron
d)
all have the same de Broglie wavelength
|
|
Gaurav Kumar answered |
De Broglie wavelength = h/mv,
where h = plancks constant, mv = momentum.
As they move with same momentum, de Broglie wavelength remains constant for all.
where h = plancks constant, mv = momentum.
As they move with same momentum, de Broglie wavelength remains constant for all.
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4 eV. The stopping potential is- a)2 V
- b)6 V
- c)4 V
- d)10 V
Correct answer is option 'C'. Can you explain this answer?
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4 eV. The stopping potential is
a)
2 V
b)
6 V
c)
4 V
d)
10 V
|
|
Rajeev Saxena answered |
Stopping potential is nothing but the maximum kinetic energy of electros which get emitted during photoelectric effect.
So the stopping potential = maximum kinetic energy = 4eV
When ultraviolet radiation is incident on asurface, no photoelectrons are emitted. If asecond beam causes photoelectrons to beejected, it may consists of [2002]- a)infra-red waves
- b)X-rays
- c)visible light rays
- d)radio waves
Correct answer is option 'B'. Can you explain this answer?
When ultraviolet radiation is incident on asurface, no photoelectrons are emitted. If asecond beam causes photoelectrons to beejected, it may consists of [2002]
a)
infra-red waves
b)
X-rays
c)
visible light rays
d)
radio waves

|
Anirudh Datta answered |
Energy of photon of X-rays is more than
energy of photon of ultraviolet rays. Because
frequency of X rays is more than ultraviolet
rays.
energy of photon of ultraviolet rays. Because
frequency of X rays is more than ultraviolet
rays.
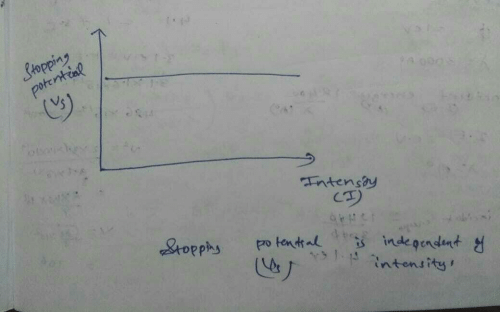
In various experiments on photo electricity the stopping potential for a given frequency of the incident radiation- a)is independent of the radiation intensity
- b)is inversely proportional to radiation intensity
- c)is proportional to radiation intensity
- d)none
Correct answer is option 'A'. Can you explain this answer?
In various experiments on photo electricity the stopping potential for a given frequency of the incident radiation
a)
is independent of the radiation intensity
b)
is inversely proportional to radiation intensity
c)
is proportional to radiation intensity
d)
none
|
|
Shreya Singh answered |
If the voltage across the electrodes of a cathode ray tube is 500 volts then energy gained by the electrons is- a)7 ×10-17 J
- b)8 ×10−17 J
- c)6 ×10−17 J
- d)9 ×10−17 J
Correct answer is option 'B'. Can you explain this answer?
If the voltage across the electrodes of a cathode ray tube is 500 volts then energy gained by the electrons is
a)
7 ×10-17 J
b)
8 ×10−17 J
c)
6 ×10−17 J
d)
9 ×10−17 J
|
|
Om Desai answered |
Voltage across the electrodes of a cathode ray gun, V=500V
Charge of the electron=1.6x10-19
Energy=eV
E= 1.6x10-19 x 500
E=800 x 10-19 J
E=8 x 10-17 J
Charge of the electron=1.6x10-19
Energy=eV
E= 1.6x10-19 x 500
E=800 x 10-19 J
E=8 x 10-17 J
Photons can be- a)scattered
- b)deflected by magnetic fields
- c)deflected by electric fields
- d)none
Correct answer is option 'A'. Can you explain this answer?
Photons can be
a)
scattered
b)
deflected by magnetic fields
c)
deflected by electric fields
d)
none
|
|
Rajeev Saxena answered |
As this electron changes orbit, its energy is reduced, and the excess energy is given off in the form of a photon, called a “characteristic photon.” In pair production, photon energies greater than 1.02 MeV interact with the strong electric field of the nucleus and lose all incident energy.
Light of two different frequencies whose photons have energies of 1eV and 2.5 eV respectively successively illuminate a metal of work function 0.5eV. The ratio of maximum speed of emitted electrons is- a)1:5
- b)1:4
- c)1:3
- d)1:2
Correct answer is option 'D'. Can you explain this answer?
Light of two different frequencies whose photons have energies of 1eV and 2.5 eV respectively successively illuminate a metal of work function 0.5eV. The ratio of maximum speed of emitted electrons is
a)
1:5
b)
1:4
c)
1:3
d)
1:2
|
|
Shreya Gupta answered |
In Photoelectric effect- a)electrical energy is converted magnetic field energy
- b)electrical energy is converted into light energy
- c)light is converted into electrical energy
- d)electrical energy is converted into heat
Correct answer is option 'C'. Can you explain this answer?
In Photoelectric effect
a)
electrical energy is converted magnetic field energy
b)
electrical energy is converted into light energy
c)
light is converted into electrical energy
d)
electrical energy is converted into heat
|
|
Nikita Singh answered |
Photoelectric cell or photocell, device whose electrical characteristics (e.g., current, voltage, or resistance) vary when light is incident upon it. The most common type consists of two electrodes separated by a light-sensitive semiconductor material.
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Electrons emitted in this manner can be called photoelectrons.
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Electrons emitted in this manner can be called photoelectrons.
A photon is- a)a positive charged particle
- b)a quantum of light energy
- c)an instrument for measuring light intensity
- d)a quantum of matter
Correct answer is option 'B'. Can you explain this answer?
A photon is
a)
a positive charged particle
b)
a quantum of light energy
c)
an instrument for measuring light intensity
d)
a quantum of matter
|
|
Rajat Patel answered |
The photon is a type of elementary particle, the quantum of the electromagnetic field including electromagnetic radiation such as light, and the force carrier for the electromagnetic force (even when static via virtual particles). ... The quanta in a light wave are not spatially localized.
in photoelectric effect, the photoelectric current- a)both on intensity and frequency of incident beam
- b)increases when frequency of incident photons increases
- c)decreases when frequency of incident photons increases
- d)does not depend on photon frequency but only on intensity of incident
Correct answer is option 'D'. Can you explain this answer?
in photoelectric effect, the photoelectric current
a)
both on intensity and frequency of incident beam
b)
increases when frequency of incident photons increases
c)
decreases when frequency of incident photons increases
d)
does not depend on photon frequency but only on intensity of incident
|
|
Arun Khanna answered |
In photoelectric effect,when light incident on metal surface the no. Of electron emitted is depends on intensity of light and speed of Electron is depends on frequency of incidents light. Photoelectric current is due to the ejection of photo electrons.
Photoelectric effect is- a)emission of electrons by glass when illuminated by light of suitable frequency
- b)emission of electrons by metals when heated
- c)emission of electrons by insulators when illuminated by light of suitable frequency
- d)emission of electrons by metals when illuminated by light of suitable frequency
Correct answer is option 'D'. Can you explain this answer?
Photoelectric effect is
a)
emission of electrons by glass when illuminated by light of suitable frequency
b)
emission of electrons by metals when heated
c)
emission of electrons by insulators when illuminated by light of suitable frequency
d)
emission of electrons by metals when illuminated by light of suitable frequency

|
Rising Star answered |
Photoelectric effect is emission of electron from metal when illuminated by light of suitable frequency.it is known as drift frequency.
A photo-cell employs photoelectric effect toconvert [2006]- a)change in the intensity of illumination intoa change in photoelectric current
- b)change in the intensity of illumination intoa change in the work function of thephotocathode
- c)change in the frequency of light into achange in the electric current
- d)change in the frequency of light into achange in electric voltage
Correct answer is option 'A'. Can you explain this answer?
A photo-cell employs photoelectric effect toconvert [2006]
a)
change in the intensity of illumination intoa change in photoelectric current
b)
change in the intensity of illumination intoa change in the work function of thephotocathode
c)
change in the frequency of light into achange in the electric current
d)
change in the frequency of light into achange in electric voltage

|
Ayush Chavan answered |
A photo-cell employs photoelectric effect to
convert light energy into photoelectric
current.
convert light energy into photoelectric
current.
A source of light is placed at a distance of 50 cm from a photocell and the stopping potential is found to be V0. If the distance between the lightsource and photocell is made 25 cm, the newstopping potential will be [NEET Kar. 2013]- a)2V0
- b)V0/2
- c)V0
- d)4V0
Correct answer is option 'C'. Can you explain this answer?
A source of light is placed at a distance of 50 cm from a photocell and the stopping potential is found to be V0. If the distance between the lightsource and photocell is made 25 cm, the newstopping potential will be [NEET Kar. 2013]
a)
2V0
b)
V0/2
c)
V0
d)
4V0

|
Anand Jain answered |
Since, stopping potential is independent
of distance hence new stopping potential
will remain unchanged i.e., new stopping
potential = V0.
of distance hence new stopping potential
will remain unchanged i.e., new stopping
potential = V0.
If the work function of a material is 2eV, then minimum frequency of light required to emit photo-electrons is - a)5.0 ×1015Hz
- b)4.6 ×1015Hz
- c)4.6 ×1014Hz
- d)5.0 ×1014Hz
Correct answer is option 'C'. Can you explain this answer?
If the work function of a material is 2eV, then minimum frequency of light required to emit photo-electrons is
a)
5.0 ×1015Hz
b)
4.6 ×1015Hz
c)
4.6 ×1014Hz
d)
5.0 ×1014Hz
|
|
Lavanya Menon answered |
Φ= hνλ => 2eV= 6.626 x 10-34 x ν
2 x 1.6 x 10-19= 6.626 x 10-34 x ν
On solving we get ν = 4.6 x 1014 Hz.
In a discharge tube ionization of enclosed gas isproduced due to collisions between [2006]- a)negative electrons and neutral atoms /molecules
- b)photons and neutral atoms /molecules
- c)neutral gas atoms/molecules
- d)positive ions and neutral atoms/molecules
Correct answer is option 'A'. Can you explain this answer?
In a discharge tube ionization of enclosed gas isproduced due to collisions between [2006]
a)
negative electrons and neutral atoms /molecules
b)
photons and neutral atoms /molecules
c)
neutral gas atoms/molecules
d)
positive ions and neutral atoms/molecules

|
Krish Patel answered |
When electrons emitted from cathode collide
with gas molecules or atoms, they knock out
outer electrons and produce positively
charged ions. They become part of positive
rays.
with gas molecules or atoms, they knock out
outer electrons and produce positively
charged ions. They become part of positive
rays.
In the above experimental set up for studying photoelectric effect, if keeping the frequency of the incident radiation and the accelerating potential fixed, the intensity of light is varied, then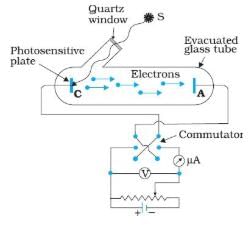
- a)photocurrent decreases nonlinearly with intensity
- b)photocurrent decreases linearly with intensity
- c)photocurrent remains same with intensity
- d)photocurrent increases linearly with intensity
Correct answer is option 'D'. Can you explain this answer?
In the above experimental set up for studying photoelectric effect, if keeping the frequency of the incident radiation and the accelerating potential fixed, the intensity of light is varied, then

a)
photocurrent decreases nonlinearly with intensity
b)
photocurrent decreases linearly with intensity
c)
photocurrent remains same with intensity
d)
photocurrent increases linearly with intensity
|
|
Nikita Singh answered |
The number of electrons emitted per second is observed to be directly proportional to the intensity of light. “Ok, so light is a wave and has energy. It takes electrons out of a metal, what is so special about that!” First of all, when the intensity of light is increased, we should see an increase in the photocurrent (number of photoelectrons emitted). Right?
As we see, this only happens above a specific value of frequency, known as the threshold frequency. Below this threshold frequency, the intensity of light has no effect on the photocurrent! In fact, there is no photocurrent at all, however high the intensity of light is.
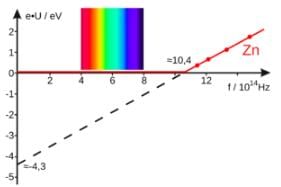
The graph between the photoelectric current and the intensity of light is a straight line when the frequency of light used is above a specific minimum threshold value.
As we see, this only happens above a specific value of frequency, known as the threshold frequency. Below this threshold frequency, the intensity of light has no effect on the photocurrent! In fact, there is no photocurrent at all, however high the intensity of light is.

The graph between the photoelectric current and the intensity of light is a straight line when the frequency of light used is above a specific minimum threshold value.
Energy of a photon of green light of wavelength 5500 is (given: h = 6.62 ×10−34Js−1) approximately- a)3.01eV
- b)2.81ev
- c)2.26 eV
- d)2.93 eV
Correct answer is 'C'. Can you explain this answer?
Energy of a photon of green light of wavelength 5500 is (given: h = 6.62 ×10−34Js−1) approximately
a)
3.01eV
b)
2.81ev
c)
2.26 eV
d)
2.93 eV
|
|
Rajesh Gupta answered |
As we know,
the formula for energy of photon in terms of wavelength is,, E = hc/ λ
where, E = energy of photon
h = Planck's constant =
= 6.63×10-34 Js
c = speed of light
= 3×108 m/s
and lambda = wavelength
so,
E = [6.63×10-34 J. s×3×108 m/s]/5500×10-10m
= [0.36×10-26]/10-8
= 0.36×10-18
= 3.6×10-19 J
Or, 2.26ev
the formula for energy of photon in terms of wavelength is,, E = hc/ λ
where, E = energy of photon
h = Planck's constant =
= 6.63×10-34 Js
c = speed of light
= 3×108 m/s
and lambda = wavelength
so,
E = [6.63×10-34 J. s×3×108 m/s]/5500×10-10m
= [0.36×10-26]/10-8
= 0.36×10-18
= 3.6×10-19 J
Or, 2.26ev
The photoelectric threshold frequency of a metal is f0. When light of frequency 4f0is incident on the metal, the maximum K.E. of the emitted electron is- a)2 hf0
- b)4 hf0
- c)3 hf0
- d)hf0/ 2
Correct answer is option 'C'. Can you explain this answer?
The photoelectric threshold frequency of a metal is f0. When light of frequency 4f0is incident on the metal, the maximum K.E. of the emitted electron is
a)
2 hf0
b)
4 hf0
c)
3 hf0
d)
hf0/ 2
|
|
Hansa Sharma answered |
The maximum kinetic energy of the emitted electrons is given by
Kmax=hυ−ϕ0=h(4υ)−h(υ)=3hυ
Kmax=hυ−ϕ0=h(4υ)−h(υ)=3hυ
The work function of metal is 1 eV. Light of wavelength 3000 Å is incident on this metal surface. The velocity of emitted photo-electrons will be- a) 10 m/sec
- b)1 × 103 m / sec
- c)1 × 104 m / sec
- d)1× 106 m / sec
Correct answer is option 'D'. Can you explain this answer?
The work function of metal is 1 eV. Light of wavelength 3000 Å is incident on this metal surface. The velocity of emitted photo-electrons will be
a)
10 m/sec
b)
1 × 103 m / sec
c)
1 × 104 m / sec
d)
1× 106 m / sec
|
|
Sai Mehta answered |
Understanding the Problem
The work function of the metal is given as 1 eV, and light of wavelength 3000 Å (Angstroms) is incident on it. We need to find the velocity of the emitted photoelectrons.
Key Concepts
- Work Function (Φ): The minimum energy required to remove an electron from the surface of a metal, given in electron volts (eV).
- Energy of Photons (E): The energy of the incident light can be calculated using the formula:
E = hc/λ
where:
- h = Planck's constant (4.135667696 × 10^-15 eV·s)
- c = Speed of light (3 × 10^8 m/s)
- λ = Wavelength (in meters)
Calculating Energy of Incident Light
1. Convert Wavelength:
3000 Å = 3000 × 10^-10 m = 3 × 10^-7 m
2. Calculate Energy (E):
E = (4.135667696 × 10^-15 eV·s) × (3 × 10^8 m/s) / (3 × 10^-7 m)
E = 4.135667696 eV
Photoelectric Effect
- Kinetic Energy (KE): The kinetic energy of the emitted electrons is given by:
KE = E - Φ
KE = 4.135667696 eV - 1 eV = 3.135667696 eV
Calculating Velocity of Electrons
- Using Kinetic Energy Formula:
KE = (1/2)mv², where m is the mass of an electron (approximately 9.11 × 10^-31 kg).
- Rearranging gives:
v = sqrt(2 * KE / m)
- Converting KE to Joules (1 eV = 1.6 × 10^-19 J):
KE = 3.135667696 eV × 1.6 × 10^-19 J/eV = 5.016 e-19 J
- Now, substituting the values:
v = sqrt(2 * 5.016 e-19 J / 9.11 e-31 kg)
v ≈ 1 × 10^6 m/s
Conclusion
Thus, the velocity of the emitted photoelectrons is approximately 1 × 10^6 m/s, confirming that the correct answer is option 'D'.
The work function of the metal is given as 1 eV, and light of wavelength 3000 Å (Angstroms) is incident on it. We need to find the velocity of the emitted photoelectrons.
Key Concepts
- Work Function (Φ): The minimum energy required to remove an electron from the surface of a metal, given in electron volts (eV).
- Energy of Photons (E): The energy of the incident light can be calculated using the formula:
E = hc/λ
where:
- h = Planck's constant (4.135667696 × 10^-15 eV·s)
- c = Speed of light (3 × 10^8 m/s)
- λ = Wavelength (in meters)
Calculating Energy of Incident Light
1. Convert Wavelength:
3000 Å = 3000 × 10^-10 m = 3 × 10^-7 m
2. Calculate Energy (E):
E = (4.135667696 × 10^-15 eV·s) × (3 × 10^8 m/s) / (3 × 10^-7 m)
E = 4.135667696 eV
Photoelectric Effect
- Kinetic Energy (KE): The kinetic energy of the emitted electrons is given by:
KE = E - Φ
KE = 4.135667696 eV - 1 eV = 3.135667696 eV
Calculating Velocity of Electrons
- Using Kinetic Energy Formula:
KE = (1/2)mv², where m is the mass of an electron (approximately 9.11 × 10^-31 kg).
- Rearranging gives:
v = sqrt(2 * KE / m)
- Converting KE to Joules (1 eV = 1.6 × 10^-19 J):
KE = 3.135667696 eV × 1.6 × 10^-19 J/eV = 5.016 e-19 J
- Now, substituting the values:
v = sqrt(2 * 5.016 e-19 J / 9.11 e-31 kg)
v ≈ 1 × 10^6 m/s
Conclusion
Thus, the velocity of the emitted photoelectrons is approximately 1 × 10^6 m/s, confirming that the correct answer is option 'D'.
Monochromatic radiation emitted when electronon hydrogen atom jumps from first excited tothe ground state irradiates a photosensitivematerial. The stopping potential is measured tobe 3.57 V. The threshold frequency of the materialsis : [2012]
- a)4 × 1015 Hz
- b)5 × 1015 Hz
- c)1.6 × 1015` Hz
- d)2.5 × 1015 Hz
Correct answer is option 'C'. Can you explain this answer?
Monochromatic radiation emitted when electronon hydrogen atom jumps from first excited tothe ground state irradiates a photosensitivematerial. The stopping potential is measured tobe 3.57 V. The threshold frequency of the materialsis : [2012]
a)
4 × 1015 Hz
b)
5 × 1015 Hz
c)
1.6 × 1015` Hz
d)
2.5 × 1015 Hz

|
Soumya Ahuja answered |
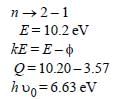
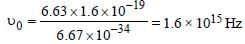
A particle of mass 1 mg has the same wavelengthas an electron moving with a velocity of 3×106 ms–1. The velocity of the particle is: [2008]
(mass of electron = 9.1×10–31 kg)- a)2.7× 10–18 ms–1
- b)9 × 10–2 ms–1
- c)3 × 10–31 ms–1
- d)2.7×10–21 ms–1
Correct answer is option 'D'. Can you explain this answer?
A particle of mass 1 mg has the same wavelengthas an electron moving with a velocity of 3×106 ms–1. The velocity of the particle is: [2008]
(mass of electron = 9.1×10–31 kg)
(mass of electron = 9.1×10–31 kg)
a)
2.7× 10–18 ms–1
b)
9 × 10–2 ms–1
c)
3 × 10–31 ms–1
d)
2.7×10–21 ms–1

|
Soumya Ahuja answered |
Wavelength of
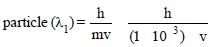
where v is the velocity of the particle.
Wave length of electron
Wave length of electron
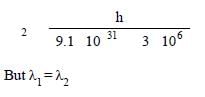
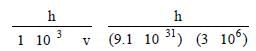
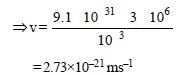
Two radiations of photons energies 1 eV and 2.5 eV, successively illuminate a photosensitivemetallic surface of work function 0.5 eV. The ratioof the maximum speeds of the emitted electronsis : [2012M]- a)1 : 4
- b)1 : 2
- c)1 : 1
- d)1 : 5
Correct answer is option 'B'. Can you explain this answer?
Two radiations of photons energies 1 eV and 2.5 eV, successively illuminate a photosensitivemetallic surface of work function 0.5 eV. The ratioof the maximum speeds of the emitted electronsis : [2012M]
a)
1 : 4
b)
1 : 2
c)
1 : 1
d)
1 : 5

|
Mrinalini Bajaj answered |
According to Einsten’s photoelectric effect,
the K.E. of the radiated electrons
the K.E. of the radiated electrons
K.Emax = E-W
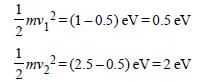
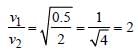
The work function of a surface of aphotosensitive material is 6.2 eV. The wavelengthof incident radiation for which the stoppingpotential is 5 V lies in the: [2008]- a)Ultraviolet region
- b)Visible region
- c)Infrared region
- d)X-ray region
Correct answer is option 'A'. Can you explain this answer?
The work function of a surface of aphotosensitive material is 6.2 eV. The wavelengthof incident radiation for which the stoppingpotential is 5 V lies in the: [2008]
a)
Ultraviolet region
b)
Visible region
c)
Infrared region
d)
X-ray region
|
|
Shail Chawla answered |
Work Function and Stopping Potential
Work Function:
The amount of energy required to remove an electron from the surface of a metal is called the work function. It is denoted by Φ and is measured in electron-volts (eV).
Stopping Potential:
When photoelectrons are emitted from the surface of a metal, they are accelerated by an electric field towards a collector electrode. The potential difference required to stop these photoelectrons from reaching the collector electrode is called stopping potential.
Relation between Work Function and Stopping Potential
The stopping potential is directly proportional to the frequency of the incident radiation and inversely proportional to the work function of the metal surface.
The equation relating stopping potential, frequency and work function is:
V0 = (h / e) x (ν - ν0)
where V0 is the stopping potential, h is Planck's constant, e is the charge of an electron, ν is the frequency of the incident radiation and ν0 is the threshold frequency (minimum frequency) required to eject an electron from the metal surface.
Solution
Given, work function of the photosensitive material = 6.2 eV and stopping potential = 5 V.
Let's calculate the threshold frequency ν0 using the work function:
ν0 = Φ / h = 6.2 / 4.14 x 10^-15 = 1.50 x 10^15 Hz
Now, using the equation of stopping potential, we can find the frequency of the incident radiation:
V0 = (h / e) x (ν - ν0)
5 = (4.14 x 10^-15 / 1.6 x 10^-19) x (ν - 1.50 x 10^15)
ν - 1.50 x 10^15 = 1.22 x 10^15 Hz
ν = 2.72 x 10^15 Hz
The frequency of the incident radiation is 2.72 x 10^15 Hz, which corresponds to a wavelength of about 110 nm. This wavelength lies in the ultraviolet region of the electromagnetic spectrum.
Therefore, the correct answer is option 'A': Ultraviolet region.
Work Function:
The amount of energy required to remove an electron from the surface of a metal is called the work function. It is denoted by Φ and is measured in electron-volts (eV).
Stopping Potential:
When photoelectrons are emitted from the surface of a metal, they are accelerated by an electric field towards a collector electrode. The potential difference required to stop these photoelectrons from reaching the collector electrode is called stopping potential.
Relation between Work Function and Stopping Potential
The stopping potential is directly proportional to the frequency of the incident radiation and inversely proportional to the work function of the metal surface.
The equation relating stopping potential, frequency and work function is:
V0 = (h / e) x (ν - ν0)
where V0 is the stopping potential, h is Planck's constant, e is the charge of an electron, ν is the frequency of the incident radiation and ν0 is the threshold frequency (minimum frequency) required to eject an electron from the metal surface.
Solution
Given, work function of the photosensitive material = 6.2 eV and stopping potential = 5 V.
Let's calculate the threshold frequency ν0 using the work function:
ν0 = Φ / h = 6.2 / 4.14 x 10^-15 = 1.50 x 10^15 Hz
Now, using the equation of stopping potential, we can find the frequency of the incident radiation:
V0 = (h / e) x (ν - ν0)
5 = (4.14 x 10^-15 / 1.6 x 10^-19) x (ν - 1.50 x 10^15)
ν - 1.50 x 10^15 = 1.22 x 10^15 Hz
ν = 2.72 x 10^15 Hz
The frequency of the incident radiation is 2.72 x 10^15 Hz, which corresponds to a wavelength of about 110 nm. This wavelength lies in the ultraviolet region of the electromagnetic spectrum.
Therefore, the correct answer is option 'A': Ultraviolet region.
Monochromatic light of frequency 6.0 × 1014 Hzis produced by a laser. The power emitted is 2 × 10–3 ω. The number of photons emitted, onthe average, by the sources per second is [2007]- a)5 × l 016
- b)5 × 1017
- c)5 × 1014
- d)5 × 1015
Correct answer is option 'D'. Can you explain this answer?
Monochromatic light of frequency 6.0 × 1014 Hzis produced by a laser. The power emitted is 2 × 10–3 ω. The number of photons emitted, onthe average, by the sources per second is [2007]
a)
5 × l 016
b)
5 × 1017
c)
5 × 1014
d)
5 × 1015

|
Sneha Basak answered |
Since p = nhv
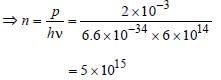
A photoelectric cell is illuminated by a pointsource of light 1m away. When the source isshifted to 2m then [2003]- a)number of electrons emitted is a quarter ofthe initial number
- b)each emitted electron carries one quarter ofthe initial energy
- c)number of electrons emitted is half the initialnumber
- d)each emitted electron carries half the initialenergy
Correct answer is option 'A'. Can you explain this answer?
A photoelectric cell is illuminated by a pointsource of light 1m away. When the source isshifted to 2m then [2003]
a)
number of electrons emitted is a quarter ofthe initial number
b)
each emitted electron carries one quarter ofthe initial energy
c)
number of electrons emitted is half the initialnumber
d)
each emitted electron carries half the initialenergy

|
Diya Datta answered |
Power ∝ No. of electrons emitted (N)
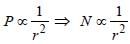
An X-ray tube is operating at 50 kV and 20 mA. The target material of the tube has a mass of 1.0 kg and specific heat 495 J kg–1°C–1. One percent of the supplied electric power is converted into X-rays and the entire remaining energy goes into heating the target. Then- a)A suitable target material must have a high melting temperature
- b)A suitable target material must have low thermal conductivity
- c)The average rate of rise of temperature of target would be 2 °C/s
- d)The minimum wavelength of the X-rays emitted is about 0.25 x 10-10 m
Correct answer is option 'A,C,D'. Can you explain this answer?
An X-ray tube is operating at 50 kV and 20 mA. The target material of the tube has a mass of 1.0 kg and specific heat 495 J kg–1°C–1. One percent of the supplied electric power is converted into X-rays and the entire remaining energy goes into heating the target. Then
a)
A suitable target material must have a high melting temperature
b)
A suitable target material must have low thermal conductivity
c)
The average rate of rise of temperature of target would be 2 °C/s
d)
The minimum wavelength of the X-rays emitted is about 0.25 x 10-10 m

|
Mohit Rajpoot answered |
P = VI = 50 × 103 × 20 × 10-3 = 1000 W
Power converted into heat = 990 W
MSΔT = 990 ⇒ ΔT = 2oc/sec
Now hc/1min = eV ⇒ hc/eV = 0.248 × 10-10m
Power converted into heat = 990 W
MSΔT = 990 ⇒ ΔT = 2oc/sec
Now hc/1min = eV ⇒ hc/eV = 0.248 × 10-10m
The X-ray wavelength of Lα line of platinum (Z = 78) is 1.30 Å. The X-ray wavelength of Lα line of Molybdenum (Z = 42) is- a)5.41 Å
- b)4.20 Å
- c)2.70 Å
- d)1.35 Å
Correct answer is option 'A'. Can you explain this answer?
The X-ray wavelength of Lα line of platinum (Z = 78) is 1.30 Å. The X-ray wavelength of Lα line of Molybdenum (Z = 42) is
a)
5.41 Å
b)
4.20 Å
c)
2.70 Å
d)
1.35 Å
|
|
Arya Basu answered |
Understanding X-ray Wavelengths
X-ray wavelengths are influenced by the atomic number (Z) of the elements. The Lα line corresponds to transitions in the electron shells of atoms, specifically from the L to K shell.
Relation between Atomic Number and Wavelength
- The wavelength of X-rays decreases as the atomic number increases.
- For heavy elements like platinum (Z = 78) and molybdenum (Z = 42), the relationship can be analyzed using empirical formulas or trends observed in X-ray production.
Given Data
- Platinum (Pt): Z = 78, Wavelength (λ) = 1.30 Å
- Molybdenum (Mo): Z = 42, Wavelength (λ) = ?
Using the Empirical Wavelength Relation
- The trend observed is that the wavelength can be approximately related to the atomic number using a formula:
λ ∝ 1/Z^(n) for some constant n (where n typically varies between 1 and 2).
- For the Lα line, we can use a simplified version of this relation to estimate the wavelength for molybdenum based on the known wavelength for platinum.
Calculating the Wavelength for Molybdenum
- By substituting the values into the relationship, we estimate:
λ_Mo = λ_Pt * (Z_Pt / Z_Mo)^n
- Using n = 2 (a common approximation for Lα lines), we find that the wavelength for molybdenum shifts considerably due to its lower atomic number.
Conclusion
- This approximation leads to a wavelength of approximately 5.41 Å for molybdenum, making option 'A' the correct answer. This calculation reflects the expected inverse relation between atomic number and X-ray wavelength, confirming that heavier elements emit shorter wavelengths.
X-ray wavelengths are influenced by the atomic number (Z) of the elements. The Lα line corresponds to transitions in the electron shells of atoms, specifically from the L to K shell.
Relation between Atomic Number and Wavelength
- The wavelength of X-rays decreases as the atomic number increases.
- For heavy elements like platinum (Z = 78) and molybdenum (Z = 42), the relationship can be analyzed using empirical formulas or trends observed in X-ray production.
Given Data
- Platinum (Pt): Z = 78, Wavelength (λ) = 1.30 Å
- Molybdenum (Mo): Z = 42, Wavelength (λ) = ?
Using the Empirical Wavelength Relation
- The trend observed is that the wavelength can be approximately related to the atomic number using a formula:
λ ∝ 1/Z^(n) for some constant n (where n typically varies between 1 and 2).
- For the Lα line, we can use a simplified version of this relation to estimate the wavelength for molybdenum based on the known wavelength for platinum.
Calculating the Wavelength for Molybdenum
- By substituting the values into the relationship, we estimate:
λ_Mo = λ_Pt * (Z_Pt / Z_Mo)^n
- Using n = 2 (a common approximation for Lα lines), we find that the wavelength for molybdenum shifts considerably due to its lower atomic number.
Conclusion
- This approximation leads to a wavelength of approximately 5.41 Å for molybdenum, making option 'A' the correct answer. This calculation reflects the expected inverse relation between atomic number and X-ray wavelength, confirming that heavier elements emit shorter wavelengths.
A photosensitive metallic surface has workfunction, hv0. If photons of energy 2 hv0 fallon this surface, the electrons come out with amaximum velocity of 4 ×106 m/s. When thephoton energy is increased to 5 hv0, thenmaximum velocity of photoelectrons will be[2005]- a)2 ×107 m/s
- b)2 ×106 m/s
- c)8 ×106 m/s
- d)8 ×105 m/s
Correct answer is option 'C'. Can you explain this answer?
A photosensitive metallic surface has workfunction, hv0. If photons of energy 2 hv0 fallon this surface, the electrons come out with amaximum velocity of 4 ×106 m/s. When thephoton energy is increased to 5 hv0, thenmaximum velocity of photoelectrons will be
[2005]
a)
2 ×107 m/s
b)
2 ×106 m/s
c)
8 ×106 m/s
d)
8 ×105 m/s

|
Raghav Khanna answered |
We know that
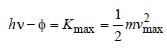
According to question
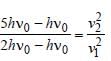
v2 = 2v1 = 2 × 4× 106 = 8 × 106 m/s.
The work functions for metals A, B and C arerespectively 1.92 eV, 2.0 eV and 5 eV.According to Einstein s equation, the metalswhich will emit photoelectrons for a radiationof wavelength 4100 Å is/are [2005]- a)none
- b)A only
- c)A and B only
- d)all three metals
Correct answer is option 'C'. Can you explain this answer?
The work functions for metals A, B and C arerespectively 1.92 eV, 2.0 eV and 5 eV.According to Einstein s equation, the metalswhich will emit photoelectrons for a radiationof wavelength 4100 Å is/are [2005]
a)
none
b)
A only
c)
A and B only
d)
all three metals
|
|
Nisha Kulkarni answered |
Nm (corresponding to an energy of 3.02 eV) are A and B.
Einstein's equation states that the energy (E) of a photon is equal to Planck's constant (h) multiplied by the frequency (ν) of the radiation: E = hν. This equation can also be written in terms of the wavelength (λ) of the radiation: E = hc/λ, where c is the speed of light.
To determine which metals will emit photoelectrons for a radiation of wavelength 4100 nm (corresponding to an energy of 3.02 eV), we need to compare the energy of the radiation to the work function of each metal. If the energy of the radiation is greater than or equal to the work function of the metal, photoelectrons will be emitted.
For metal A, the energy required to remove an electron (i.e. the work function) is 1.92 eV. The energy of the radiation is 3.02 eV, which is greater than the work function of metal A. Therefore, metal A will emit photoelectrons.
For metal B, the work function is 2.0 eV. The energy of the radiation is also greater than the work function of metal B, so it will emit photoelectrons.
For metal C, the work function is 5 eV. The energy of the radiation is less than the work function of metal C, so it will not emit photoelectrons.
Therefore, the metals that will emit photoelectrons for a radiation of wavelength 4100 nm are A and B.
Einstein's equation states that the energy (E) of a photon is equal to Planck's constant (h) multiplied by the frequency (ν) of the radiation: E = hν. This equation can also be written in terms of the wavelength (λ) of the radiation: E = hc/λ, where c is the speed of light.
To determine which metals will emit photoelectrons for a radiation of wavelength 4100 nm (corresponding to an energy of 3.02 eV), we need to compare the energy of the radiation to the work function of each metal. If the energy of the radiation is greater than or equal to the work function of the metal, photoelectrons will be emitted.
For metal A, the energy required to remove an electron (i.e. the work function) is 1.92 eV. The energy of the radiation is 3.02 eV, which is greater than the work function of metal A. Therefore, metal A will emit photoelectrons.
For metal B, the work function is 2.0 eV. The energy of the radiation is also greater than the work function of metal B, so it will emit photoelectrons.
For metal C, the work function is 5 eV. The energy of the radiation is less than the work function of metal C, so it will not emit photoelectrons.
Therefore, the metals that will emit photoelectrons for a radiation of wavelength 4100 nm are A and B.
Chapter doubts & questions for Dual Nature of Radiation and Matter - Physics Class 12 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Dual Nature of Radiation and Matter - Physics Class 12 in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup