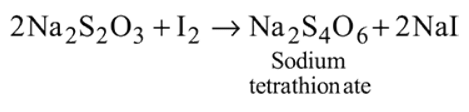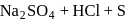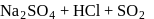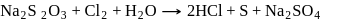All Exams >
JEE >
Chemistry for JEE Main & Advanced >
All Questions
All questions of p-Block Elements for JEE Exam
Which of the following statements is true?a)The atomic radius of Ga is less than B.
b)The atomic radius of Ga is more than Al.c)The atomic radius of Ga is less than Al.d)The atomic radius of Ga is equal to AlCorrect answer is option 'C'. Can you explain this answer?
b)The atomic radius of Ga is more than Al.
c)The atomic radius of Ga is less than Al.
d)The atomic radius of Ga is equal to Al
Correct answer is option 'C'. Can you explain this answer?
|
|
Neha Joshi answered |
The atomic radius of the Ga is less than Al because of poor screening effect. The atomic radius of Ga is slightly lesser than of Al because in going from Al to Ga, the electrons have already occupied 3d sub shell in Ga
The exhibition of highest co-ordination number depends on the availability of vacant orbitals in the central atom. Which of the following elements is not likely to act as central atom in MF3-6?- a)B
- b)Al
- c)Ga
- d)In
Correct answer is option 'A'. Can you explain this answer?
The exhibition of highest co-ordination number depends on the availability of vacant orbitals in the central atom. Which of the following elements is not likely to act as central atom in MF3-6?
a)
B
b)
Al
c)
Ga
d)
In
|
|
Anjana Sharma answered |
The element M in the complex ion MF6^3- has a coordination number of six. Since B has only s- and p-orbitals and no d – orbitals, therefore, at the maximum it can show a coordination number of 4. Thus, B cannot form complex of the type MF6^3-, i.e., option (a) is correct.
Which of the following elements exist as liquid in summer among group 13 elements?
- a) Tl
- b) Al
- c) B
- d) Ga
Correct answer is option 'D'. Can you explain this answer?
Which of the following elements exist as liquid in summer among group 13 elements?
a)
Tlb)
Alc)
Bd)
Ga|
|
Krishna Iyer answered |
Galium can occur in liquid state if the room is above 29.76C which is its melting point. So,option d is correct
The maximum oxidation state shown by a p-block element is equal to the:- a)Total number of valence electrons (i.e., the sum of the s and p-electrons)
- b)Total number of s electrons
- c)Total number of p electrons
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
The maximum oxidation state shown by a p-block element is equal to the:
a)
Total number of valence electrons (i.e., the sum of the s and p-electrons)
b)
Total number of s electrons
c)
Total number of p electrons
d)
None of these
|
|
Preeti Khanna answered |
- The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds.
- It also determines the ability of an atom to oxidize (to lose electrons) or to reduce (to gain electrons) other atoms or species.
- They should release the total valence electrons to attain stability, so the maximum possible oxidation state is the number of valence electrons.
The order of ionization enthalpy for B, Al and Ga is:- a)B>Al<Ga
- b)B
- c)B>Al>Ga
- d)Ga
Correct answer is option 'A'. Can you explain this answer?
The order of ionization enthalpy for B, Al and Ga is:
a)
B>Al<Ga
b)
B
c)
B>Al>Ga
d)
Ga

|
Ayush Joshi answered |
(A) B>Al<Ga
The trend in ionization enthalpy is because there is decrease in Ionisation enthalpy from B to Al due to increase in size and from Al to Ga. Ga has more ionisation energy than Al due to uneffective screening effect.
Which of the following group 13 elements oxide is acidic in nature?- a)Al2O3
- b)B2O3
- c)Tl2O3
- d)Ga2O3
Correct answer is option 'B'. Can you explain this answer?
Which of the following group 13 elements oxide is acidic in nature?
a)
Al2O3
b)
B2O3
c)
Tl2O3
d)
Ga2O3
|
|
Naina Bansal answered |
The acidic character of oxides of group 13 decreases down the group, because non-metallic character of elements decreases down the group and metallic character increases.
And we know that oxides of metals are basic in nature and oxides of non-metals are acidic in nature. So acidic character of oxides of group 13 decreases down the group.
For example boron (1st element of group 13) is non-metal, so its oxide is acidic.
Aluminum (2nd element of group 13) shows characteristics of both metal and non-metal, so its oxide shows amphoteric nature.
As we go down the group, indium and thalium (4th and 5th element of group 13) show metallic properties, so their oxides are basic.
Which one of the following does not form hydrogen bonding?- a)NH3
- b)HCl
- c)H2O
- d)HF
Correct answer is option 'B'. Can you explain this answer?
Which one of the following does not form hydrogen bonding?
a)
NH3
b)
HCl
c)
H2O
d)
HF

|
Lead Academy answered |
- Hydrochloric acid (HCl) does not form hydrogen bonding.
- This is because in hydrogen bonding the bonding is formed between hydrogen and elements Nitrogen, Oxygen and Flourine.
- In Hydrochloric acid (HCl) there are no elements like Nitrogen, Oxygen and Flourine so it cannot form hydrogen bonding.
Fluorine differs from rest of the halogens in some of its properties. This is due to
- a)its smaller size and high electronegativity
- b)lack of d-orbitals
- c)low bond dissociation energy
- d)Both A and B
Correct answer is option 'D'. Can you explain this answer?
Fluorine differs from rest of the halogens in some of its properties. This is due to
a)
its smaller size and high electronegativity
b)
lack of d-orbitals
c)
low bond dissociation energy
d)
Both A and B
|
|
Mira Tiwari answered |
Explanation:
Fluorine differs from the rest of the halogens in some of its properties due to the lack of d-orbitals. The explanation for this can be understood by considering the electronic configuration and the atomic structure of fluorine.
Electronic Configuration:
Fluorine has an atomic number of 9, and its electronic configuration is 1s2 2s2 2p5. It has a completely filled 2s orbital and an incomplete 2p orbital with one electron in each of the three available 2p orbitals.
Atomic Structure:
The atomic structure of fluorine consists of a nucleus at the center, which contains 9 protons and 9 neutrons. Surrounding the nucleus are two energy levels or shells, with the first shell containing 2 electrons and the second shell containing 7 electrons.
Lack of d-orbitals:
Fluorine does not have any d-orbitals in its valence shell. The valence shell of an atom is the outermost shell that participates in chemical bonding. In the case of fluorine, the valence shell is the second shell, which contains 7 electrons in the 2s and 2p orbitals. Since the second shell does not have any d-orbitals, fluorine cannot accommodate any d-electrons.
Consequences:
The lack of d-orbitals in fluorine has several consequences that distinguish it from the rest of the halogens:
1. Size: Fluorine is the smallest halogen atom due to its smaller atomic radius. This is because the lack of d-orbitals results in a more compact electron cloud around the nucleus.
2. Electronegativity: Fluorine has the highest electronegativity among the halogens. This is because the lack of d-orbitals allows the 2p orbitals to be closer to the nucleus, leading to greater attraction for bonding electrons.
3. Bond Dissociation Energy: Fluorine has a higher bond dissociation energy compared to the other halogens. Bond dissociation energy is the energy required to break a bond in a molecule. The lack of d-orbitals in fluorine results in stronger bonds, making it more difficult to break them.
In summary, the lack of d-orbitals in fluorine's valence shell leads to its smaller size, higher electronegativity, and higher bond dissociation energy, which distinguish it from the rest of the halogens.
Fluorine differs from the rest of the halogens in some of its properties due to the lack of d-orbitals. The explanation for this can be understood by considering the electronic configuration and the atomic structure of fluorine.
Electronic Configuration:
Fluorine has an atomic number of 9, and its electronic configuration is 1s2 2s2 2p5. It has a completely filled 2s orbital and an incomplete 2p orbital with one electron in each of the three available 2p orbitals.
Atomic Structure:
The atomic structure of fluorine consists of a nucleus at the center, which contains 9 protons and 9 neutrons. Surrounding the nucleus are two energy levels or shells, with the first shell containing 2 electrons and the second shell containing 7 electrons.
Lack of d-orbitals:
Fluorine does not have any d-orbitals in its valence shell. The valence shell of an atom is the outermost shell that participates in chemical bonding. In the case of fluorine, the valence shell is the second shell, which contains 7 electrons in the 2s and 2p orbitals. Since the second shell does not have any d-orbitals, fluorine cannot accommodate any d-electrons.
Consequences:
The lack of d-orbitals in fluorine has several consequences that distinguish it from the rest of the halogens:
1. Size: Fluorine is the smallest halogen atom due to its smaller atomic radius. This is because the lack of d-orbitals results in a more compact electron cloud around the nucleus.
2. Electronegativity: Fluorine has the highest electronegativity among the halogens. This is because the lack of d-orbitals allows the 2p orbitals to be closer to the nucleus, leading to greater attraction for bonding electrons.
3. Bond Dissociation Energy: Fluorine has a higher bond dissociation energy compared to the other halogens. Bond dissociation energy is the energy required to break a bond in a molecule. The lack of d-orbitals in fluorine results in stronger bonds, making it more difficult to break them.
In summary, the lack of d-orbitals in fluorine's valence shell leads to its smaller size, higher electronegativity, and higher bond dissociation energy, which distinguish it from the rest of the halogens.
Which of the following is not correctly matched?- a)SF4−gas
- b)SeF4− liquid
- c)TeF4− solid
- d)SF6− solid
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not correctly matched?
a)
SF4−gas
b)
SeF4− liquid
c)
TeF4− solid
d)
SF6− solid

|
Learners Habitat answered |
All hexafluorides of group 16 elements are gaseous in nature.
The structure of white phosphorus is- a)Square-planar
- b)Tetrahedral
- c)Pyramidal
- d)Trigonal planar
Correct answer is option 'B'. Can you explain this answer?
The structure of white phosphorus is
a)
Square-planar
b)
Tetrahedral
c)
Pyramidal
d)
Trigonal planar
|
|
Pritam Choudhary answered |
Structure of White Phosphorus
White phosphorus (P4) is a molecular form of phosphorus that exists as a tetrahedral structure. Each phosphorus atom in the white phosphorus molecule is bonded to three other phosphorus atoms, resulting in a tetrahedral arrangement.
Explanation
Phosphorus is a chemical element with the atomic number 15 and is located in Group 15 of the periodic table. It exists in several different forms, including white, red, and black phosphorus.
White phosphorus is the most common and well-known form of phosphorus. It is a waxy, translucent solid that appears white or slightly yellow. The structure of white phosphorus consists of P4 molecules, where four phosphorus atoms are covalently bonded together in a tetrahedral arrangement.
Tetrahedral Structure
The tetrahedral structure is characterized by the arrangement of four atoms or groups of atoms around a central atom, forming a pyramid shape. In white phosphorus, each phosphorus atom is bonded to three other phosphorus atoms, resulting in a tetrahedral arrangement.
Key Points:
- White phosphorus has a tetrahedral structure.
- It consists of P4 molecules.
- Each phosphorus atom is bonded to three other phosphorus atoms.
- The tetrahedral arrangement gives white phosphorus its unique properties and reactivity.
Properties and Reactivity
White phosphorus is highly reactive and flammable. It ignites spontaneously in air at room temperature, producing a bright flame and releasing toxic fumes. This reactivity is due to the presence of weak P-P bonds in the tetrahedral structure, which can easily break and react with oxygen in the air.
White phosphorus is also known for its high toxicity and ability to form poisonous compounds. It is used in various applications, including military ammunition, pesticide production, and the synthesis of other phosphorus compounds.
Conclusion
In summary, the structure of white phosphorus is tetrahedral, with each phosphorus atom bonded to three other phosphorus atoms. The tetrahedral arrangement gives white phosphorus its unique properties and reactivity, making it a useful but highly dangerous substance.
White phosphorus (P4) is a molecular form of phosphorus that exists as a tetrahedral structure. Each phosphorus atom in the white phosphorus molecule is bonded to three other phosphorus atoms, resulting in a tetrahedral arrangement.
Explanation
Phosphorus is a chemical element with the atomic number 15 and is located in Group 15 of the periodic table. It exists in several different forms, including white, red, and black phosphorus.
White phosphorus is the most common and well-known form of phosphorus. It is a waxy, translucent solid that appears white or slightly yellow. The structure of white phosphorus consists of P4 molecules, where four phosphorus atoms are covalently bonded together in a tetrahedral arrangement.
Tetrahedral Structure
The tetrahedral structure is characterized by the arrangement of four atoms or groups of atoms around a central atom, forming a pyramid shape. In white phosphorus, each phosphorus atom is bonded to three other phosphorus atoms, resulting in a tetrahedral arrangement.
Key Points:
- White phosphorus has a tetrahedral structure.
- It consists of P4 molecules.
- Each phosphorus atom is bonded to three other phosphorus atoms.
- The tetrahedral arrangement gives white phosphorus its unique properties and reactivity.
Properties and Reactivity
White phosphorus is highly reactive and flammable. It ignites spontaneously in air at room temperature, producing a bright flame and releasing toxic fumes. This reactivity is due to the presence of weak P-P bonds in the tetrahedral structure, which can easily break and react with oxygen in the air.
White phosphorus is also known for its high toxicity and ability to form poisonous compounds. It is used in various applications, including military ammunition, pesticide production, and the synthesis of other phosphorus compounds.
Conclusion
In summary, the structure of white phosphorus is tetrahedral, with each phosphorus atom bonded to three other phosphorus atoms. The tetrahedral arrangement gives white phosphorus its unique properties and reactivity, making it a useful but highly dangerous substance.
Which of the following statements regarding ozone is not correct?- a)The oxygen-oxygen bond length in ozone is identical with that of molecular oxygen
- b)The ozone is response hybrid of two structures
- c)The ozone molecule is angular in shape
- d)Ozone is used as a germicide and disinfectant for the purification of air
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements regarding ozone is not correct?
a)
The oxygen-oxygen bond length in ozone is identical with that of molecular oxygen
b)
The ozone is response hybrid of two structures
c)
The ozone molecule is angular in shape
d)
Ozone is used as a germicide and disinfectant for the purification of air
|
|
Neha Patel answered |
The oxygen-oxygen bond length in ozone is identical with of molecular oxygen
The bond length in ozone is intermediate between those of single O-O and double O=O bonds.The bond angle is O3O3 is 117∘117∘ with O-O distance 127.8pm.
The shape of the molecule 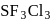 is
is- a)trigonal bipyramidal
- b)cubic
- c)octahedral
- d)tetrahedral
Correct answer is option 'C'. Can you explain this answer?
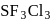 is
isa)
trigonal bipyramidal
b)
cubic
c)
octahedral
d)
tetrahedral

|
Tarun Kaushik answered |
Hybridisation is 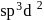 , hence octahedral
, hence octahedral
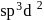 , hence octahedral
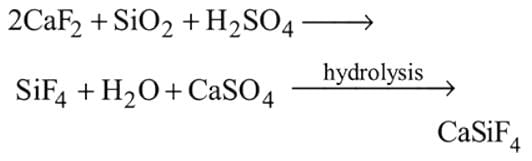
, hence octahedralA white precipitate is obtained on hydrolysis of- a)
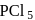
- b)
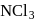
- c)
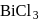
- d)
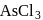
Correct answer is option 'C'. Can you explain this answer?
a)
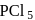
b)
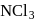
c)
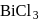
d)
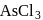

|
Learners Habitat answered |
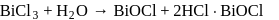
gives white ppt. which is used as white
pigment, under the name of pearl white.
Which of the following is correct about the reaction?
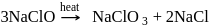
- a)It is disproportionation reaction
- b)Oxidation number of
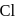 decreases as well as increases in this reaction
decreases as well as increases in this reaction - c)This reaction is used for the manufacture of halates
- d)All of these
Correct answer is option 'D'. Can you explain this answer?
Which of the following is correct about the reaction?
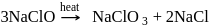
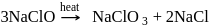
a)
It is disproportionation reaction
b)
Oxidation number of 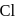 decreases as well as increases in this reaction
decreases as well as increases in this reaction
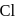 decreases as well as increases in this reaction
decreases as well as increases in this reactionc)
This reaction is used for the manufacture of halates
d)
All of these

|
Learners Habitat answered |
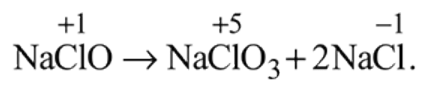
All statements are correct as evident from
the reaction.
Chapter doubts & questions for p-Block Elements - Chemistry for JEE Main & Advanced 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of p-Block Elements - Chemistry for JEE Main & Advanced in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Chemistry for JEE Main & Advanced
353 videos|587 docs|309 tests
|
Related JEE Content
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup