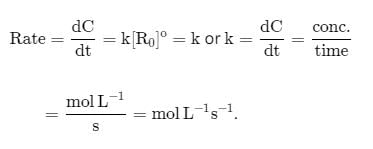All Exams >
JEE >
Chemistry for JEE Main & Advanced >
All Questions
All questions of Chemical Kinetics for JEE Exam
Half-life period of a second order reaction is
a) independent of the concentration
b) inversaly proportional to the initial conc
c) inversely proportional to conc.
d) directly proportional to the conc.
The answer is b.

|
Dr Manju Sen answered |
The correct answer is Option B.
Relation between half-life period of a reaction and initial concentration is as follows: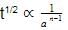
For first order reaction (Half life α a0 )
For second order reaction (Half life ∝
1/a)
For third order reaction (Half life ∝ 1a2)
Relation between half-life period of a reaction and initial concentration is as follows:
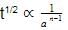
For first order reaction (Half life α a0 )
For second order reaction (Half life ∝
1/a)
For third order reaction (Half life ∝ 1a2)
For the reaction 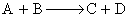 . The variation of the concentration of the products is given by curve
. The variation of the concentration of the products is given by curve 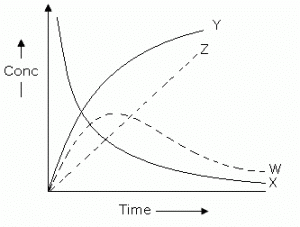
- a)Y
- b)W
- c)Z
- d)X
Correct answer is option 'A'. Can you explain this answer?
For the reaction  . The variation of the concentration of the products is given by curve
. The variation of the concentration of the products is given by curve
a)
Y
b)
W
c)
Z
d)
X

|
Divey Sethi answered |
For the reaction, A+B⟶C+D, the variation of the concentration of the products is given by the curve Y.
Initially, the product concentration is 0, then it gradually rises and reaches a maximum value. After that it remains constant.
Initially, the product concentration is 0, then it gradually rises and reaches a maximum value. After that it remains constant.
This behavior is represented by the curve Y.
The term 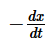 in the rate expression refers to the:
in the rate expression refers to the:- a)Increase in concentration of the reactants
- b)Average rate of the reaction
- c)Instantaneous rate of the reaction
- d)Concentration of the reactants
Correct answer is option 'C'. Can you explain this answer?
The term 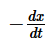 in the rate expression refers to the:
in the rate expression refers to the:
a)
Increase in concentration of the reactants
b)
Average rate of the reaction
c)
Instantaneous rate of the reaction
d)
Concentration of the reactants
|
|
Mira Sharma answered |
The term —dx/dt in the rate expression refers to theinstantaneous rate of the reaction.
The activation energy of a reaction at a given temperature is found to be 2.303 RT J mol–1. The ratio of rate constant to the Arrhenius factor is- a)0.1
- b)0.01
- c)0.001
- d)0.02
Correct answer is option 'A'. Can you explain this answer?
The activation energy of a reaction at a given temperature is found to be 2.303 RT J mol–1. The ratio of rate constant to the Arrhenius factor is
a)
0.1
b)
0.01
c)
0.001
d)
0.02
|
|
Riya Banerjee answered |
The correct answer is option A
Arrhenius equation is,
rate constant, k=Ae−Ea/RT
k=Ae−2.303RT/RT ⇒ k/A = e-2.303
On solving, we get
k/A = 10-1 or 0.1
Arrhenius equation is,
rate constant, k=Ae−Ea/RT
k=Ae−2.303RT/RT ⇒ k/A = e-2.303
On solving, we get
k/A = 10-1 or 0.1
A first order reaction is 50% completed in 20 minutes at 27°C and in 5 min at 47°C. The energy of activation of the reaction is- a)43.85 kJ/mol
- b)55.3 kJ/mol
- c)11.97 kJ/mol
- d)6.65 kJ/mol
Correct answer is option 'B'. Can you explain this answer?
A first order reaction is 50% completed in 20 minutes at 27°C and in 5 min at 47°C. The energy of activation of the reaction is
a)
43.85 kJ/mol
b)
55.3 kJ/mol
c)
11.97 kJ/mol
d)
6.65 kJ/mol
|
|
Tanuja Kapoor answered |
t1/2 = 20 min at 300 K
t’1/2 = 5 min at 320 K
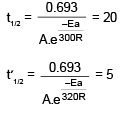
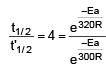
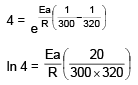
On solving, Ea = 55303.12 J
= 55.3 KJ
t’1/2 = 5 min at 320 K
On solving, Ea = 55303.12 J
= 55.3 KJ
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about [AIEEE 2011]a)64 timesb)32 timesc)24 timesd)10 timesCorrect answer is option 'B'. Can you explain this answer?
|
|
Vijay Bansal answered |
Rate at 50 degree C/(Rate at T1 degree C)
= (2)^(ΔT/T1)
= (2)^(50/10)
= 2^5
= 32
Hence, the rate of the reaction increases by 32 times.
The time for half life period of a certain reaction A → Products is 1 h. When the initial concentration of the reactant 'A' is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1, if it is a zero order reaction?- a)0.25 h
- b)1 h
- c)4 h
- d)0.5 h
Correct answer is option 'A'. Can you explain this answer?
The time for half life period of a certain reaction A → Products is 1 h. When the initial concentration of the reactant 'A' is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1, if it is a zero order reaction?
a)
0.25 h
b)
1 h
c)
4 h
d)
0.5 h

|
Shashi Kumar answered |
For a zero order reaction,
(T)half = Initial concentration/ 2× reaction constant
from this equation find out k.
also another formula for zero order reaction-
(A)t =(A)o - KT
put the value of k , (A)t=0.25 and (A)o= 0.50
you will get the answer.
(T)half = Initial concentration/ 2× reaction constant
from this equation find out k.
also another formula for zero order reaction-
(A)t =(A)o - KT
put the value of k , (A)t=0.25 and (A)o= 0.50
you will get the answer.
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about- a)64 times
- b)10 times
- c)24 times
- d)32 times
Correct answer is option 'D'. Can you explain this answer?
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about
a)
64 times
b)
10 times
c)
24 times
d)
32 times

|
Mohit Rajpoot answered |
For every 10°C rise of temperature, the rate is doubled. Thus, the temperature coefficient of the reaction = 2
When temperature is increased by 50°, rate becomes
=2(50/10) = 25 times = 32 times
When temperature is increased by 50°, rate becomes
=2(50/10) = 25 times = 32 times
A foreign substance that increase the speed of a chemical reaction is called- a)promotor
- b)catalyst
- c)moderator
- d)inhibitor
Correct answer is option 'B'. Can you explain this answer?
A foreign substance that increase the speed of a chemical reaction is called
a)
promotor
b)
catalyst
c)
moderator
d)
inhibitor
|
|
Nandini Patel answered |
Catalyst: Substances which alter the rate of a chemical reaction and themselves remain chemically and quantitatively unchanged after the reaction are known as catalysts and the phenomenon is known as catalysis.
The following mechanism has been proposed for the exothermic catalyzed complex reaction.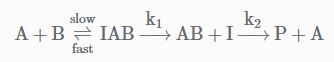 If k1 is much smaller than k2. The most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.
If k1 is much smaller than k2. The most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.- a)
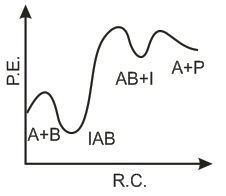
- b)
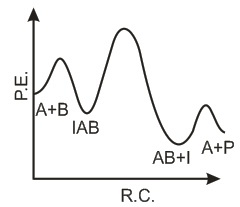
- c)
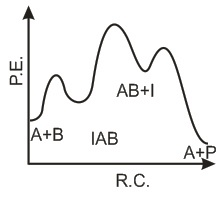
- d)
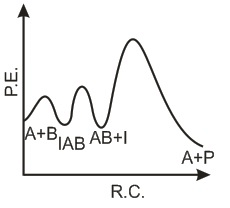
Correct answer is option 'C'. Can you explain this answer?
The following mechanism has been proposed for the exothermic catalyzed complex reaction.
If k1 is much smaller than k2. The most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.
a)

b)

c)

d)


|
Imk Pathsala answered |
Since
K1 <<< K2 = most Imp. peack will be higher
A catalyst is a substance which :- a)is always in the same phase as in the reaction
- b)alters the equilibrium in a reaction
- c)does not participate in the reaction but alters the rate of reaction
- d)participates in the reaction and provides an easier pathway for the same
Correct answer is option 'C'. Can you explain this answer?
a)
is always in the same phase as in the reaction
b)
alters the equilibrium in a reaction
c)
does not participate in the reaction but alters the rate of reaction
d)
participates in the reaction and provides an easier pathway for the same

|
Learners Habitat answered |
A catalyst does not take part in chemical reaction but it alters the rate of reaction.
. What will be the value of instantaneous rate of reaction from the graph?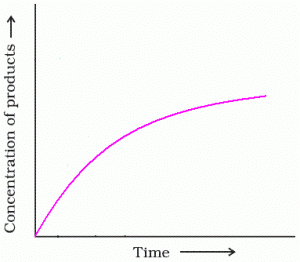
- a)rinst = 1/ Slope
- b)rinst = Slope
- c)rinst = – Slope
- d)rinst = – 1/ Slope
Correct answer is option 'B'. Can you explain this answer?
. What will be the value of instantaneous rate of reaction from the graph?

a)
rinst = 1/ Slope
b)
rinst = Slope
c)
rinst = – Slope
d)
rinst = – 1/ Slope
|
|
Preeti Iyer answered |
If you make a graph of concentration of reactant vs time, the instantaneous reaction rate at a given time is the slope of the tangent line at that point in time. It is also the value of the rate law at a specific concentration (dA/dt is rate = k [A]).
For a reaction A + B → C + D if the concentration of A is doubled without altering the concentration of B, the rate gets doubled. If the concentration of B is increased by nine times without altering the concentration of A, the rate gets tripled. The order of the reaction is - a)2
- b)1
- c)3/2
- d)4/3
Correct answer is option 'C'. Can you explain this answer?
For a reaction A + B → C + D if the concentration of A is doubled without altering the concentration of B, the rate gets doubled. If the concentration of B is increased by nine times without altering the concentration of A, the rate gets tripled. The order of the reaction is
a)
2
b)
1
c)
3/2
d)
4/3
|
|
Madhurima Mishra answered |
The reaction A → B represents a chemical reaction where substance A is converted into substance B. This is often referred to as a reactant(s) being converted into a product(s). The reaction can be written in a chemical equation format as:
A → B
A → B
In a zero-order reaction for every 10° rise of temperature, the rate is doubled. If the temperature is increased from 10°C to 100°C, the rate of the reaction will become - a)64 times
- b)128 times
- c)256 times
- d)512 times
Correct answer is option 'D'. Can you explain this answer?
In a zero-order reaction for every 10° rise of temperature, the rate is doubled. If the temperature is increased from 10°C to 100°C, the rate of the reaction will become
a)
64 times
b)
128 times
c)
256 times
d)
512 times
|
|
Ayaan Madhukar answered |
For rise in temperature, n=1
Therefore rate = 2^n = 2^1 =2
When temperature is increased from 10 degree c to 100 degree c so, change in temperature = 100 -10 = 90 degree c , therefore n = 9 , therefore rate = 2^9 = 512 times...ans...
$$Hope it's help...$$
Therefore rate = 2^n = 2^1 =2
When temperature is increased from 10 degree c to 100 degree c so, change in temperature = 100 -10 = 90 degree c , therefore n = 9 , therefore rate = 2^9 = 512 times...ans...
$$Hope it's help...$$
Identify the incorrect statement- a)ftp stands for file transfer protocol
- b)ftp uses two parallel TCP connections
- c)ftp sends its control information in band
- d)ftp sends exactly one file over the data connection
Correct answer is option 'C'. Can you explain this answer?
Identify the incorrect statement
a)
ftp stands for file transfer protocol
b)
ftp uses two parallel TCP connections
c)
ftp sends its control information in band
d)
ftp sends exactly one file over the data connection
|
|
Hansa Sharma answered |
Out-of-band controlis a characteristic of network protocols with which data control is regulated. Out-of-band control passes control data on a separate connection from main data. Protocols such asFTPuse out-of-band control. FTP uses two parallel TCP connections, one connection for sending control information (such as a request to transfer a file) and another connection foractually transferring the file. Because the control information is not sent over the same connection that the file is sent over, FTP sends control information out of band
When a graph between log K and 1/T is drawn a straight line is obtained. The temperature at which line cuts y-axis and x-axis.- a)0, Ea/2.303 R log A
- b)∞, Ea/(R ln A)
- c)0, log A
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
When a graph between log K and 1/T is drawn a straight line is obtained. The temperature at which line cuts y-axis and x-axis.
a)
0, Ea/2.303 R log A
b)
∞, Ea/(R ln A)
c)
0, log A
d)
None of these
|
|
Nandini Iyer answered |
When line cuts y axis
When it cut X -axis
log K = 0
Chemical substances speeding up the rate of chemical reaction is called as
- a)pressure
- b)concentration
- c)catalysts
- d)inhibitors
Correct answer is option 'C'. Can you explain this answer?
Chemical substances speeding up the rate of chemical reaction is called as
a)
pressure
b)
concentration
c)
catalysts
d)
inhibitors

|
Ujwal Chawla answered |
Chemical substances that speed up the rate of a chemical reaction are called catalysts. Catalysts are substances that increase the rate of a chemical reaction by providing an alternative pathway with a lower activation energy for the reaction to occur. They do not get consumed or permanently changed in the reaction and can be reused.
Catalysts work by providing an alternative reaction mechanism that has a lower activation energy. Activation energy is the energy required for a chemical reaction to occur. By lowering the activation energy, catalysts facilitate the formation of the transition state, which is the high-energy intermediate state between the reactants and the products.
There are two types of catalysts: homogeneous catalysts and heterogeneous catalysts.
Homogeneous catalysts are in the same phase as the reactants and products. They are usually soluble in the reaction mixture. Homogeneous catalysts often form complexes with reactants, which then undergo a reaction to form products. Examples of homogeneous catalysts include acids, bases, and enzymes.
Heterogeneous catalysts are in a different phase than the reactants and products. They are usually solids while the reactants and products are gases or liquids. Heterogeneous catalysts work by adsorption, where reactant molecules are adsorbed onto the catalyst surface and react to form products. Examples of heterogeneous catalysts include metal catalysts like platinum, palladium, and nickel.
Catalysts are widely used in various industries and chemical processes to increase the rate of reactions and improve efficiency. They play a crucial role in the production of fuels, chemicals, and pharmaceuticals. They can also be used to selectively promote certain reactions and inhibit others.
In conclusion, a chemical substance that speeds up the rate of a chemical reaction is called a catalyst. Catalysts work by providing an alternative pathway with a lower activation energy for the reaction to occur. They are not consumed in the reaction and can be reused. Catalysts are essential in many industries and chemical processes to increase reaction rates and improve efficiency.
Catalysts work by providing an alternative reaction mechanism that has a lower activation energy. Activation energy is the energy required for a chemical reaction to occur. By lowering the activation energy, catalysts facilitate the formation of the transition state, which is the high-energy intermediate state between the reactants and the products.
There are two types of catalysts: homogeneous catalysts and heterogeneous catalysts.
Homogeneous catalysts are in the same phase as the reactants and products. They are usually soluble in the reaction mixture. Homogeneous catalysts often form complexes with reactants, which then undergo a reaction to form products. Examples of homogeneous catalysts include acids, bases, and enzymes.
Heterogeneous catalysts are in a different phase than the reactants and products. They are usually solids while the reactants and products are gases or liquids. Heterogeneous catalysts work by adsorption, where reactant molecules are adsorbed onto the catalyst surface and react to form products. Examples of heterogeneous catalysts include metal catalysts like platinum, palladium, and nickel.
Catalysts are widely used in various industries and chemical processes to increase the rate of reactions and improve efficiency. They play a crucial role in the production of fuels, chemicals, and pharmaceuticals. They can also be used to selectively promote certain reactions and inhibit others.
In conclusion, a chemical substance that speeds up the rate of a chemical reaction is called a catalyst. Catalysts work by providing an alternative pathway with a lower activation energy for the reaction to occur. They are not consumed in the reaction and can be reused. Catalysts are essential in many industries and chemical processes to increase reaction rates and improve efficiency.
The rate constant, the activation energy and the Arrhenius parameter (A) of a chemical reaction at 25°C are 3.0 × 10-4 s-1, 104.4 kJ mol-1 and 6.0 × 10-4s-1respectively. The value of the rate constant at T → ∞ is- a)2.0 × 1018 s-1
- b) 6.0 × 1014 s-1
- c) infinity
- d)6 × 10-4 s-1
Correct answer is option 'D'. Can you explain this answer?
The rate constant, the activation energy and the Arrhenius parameter (A) of a chemical reaction at 25°C are 3.0 × 10-4 s-1, 104.4 kJ mol-1 and 6.0 × 10-4s-1respectively. The value of the rate constant at T → ∞ is
a)
2.0 × 1018 s-1
b)
6.0 × 1014 s-1
c)
infinity
d)
6 × 10-4 s-1
|
|
Gaurav Kumar answered |
At temperature = ∞
Rate constant = Arrhenious constant.
Rate constant = Arrhenious constant.
The first order rate constant k is related to temperature as log k = 15.0 - (106/T). Which of the following pair of value is correct ?- a)A = 1015 and E = 1.9 × 104 KJ
- b)A = 10-15 and E = 40 KJ
- c)A = 1015 and E = 40 KJ
- d)A = 10-15 and E = 1.9 × 104 KJ
Correct answer is option 'A'. Can you explain this answer?
The first order rate constant k is related to temperature as log k = 15.0 - (106/T). Which of the following pair of value is correct ?
a)
A = 1015 and E = 1.9 × 104 KJ
b)
A = 10-15 and E = 40 KJ
c)
A = 1015 and E = 40 KJ
d)
A = 10-15 and E = 1.9 × 104 KJ
|
|
Anagha Mukherjee answered |
Given information:
The first-order rate constant k is related to temperature as log k = 15.0 - (106/T)
To find:
The correct pair of values for A and E.
Solution:
The Arrhenius equation is given by:
k = A * e^(-E/RT)
where,
k = rate constant
A = pre-exponential factor
E = activation energy
R = universal gas constant = 8.314 J/mol*K
T = absolute temperature
Taking natural logarithm on both sides of the equation, we get:
ln k = ln A - (E/RT)
Comparing this equation with the given equation, we can see that:
ln A = 15.0
E/R = 106/T
Solving for A and E, we get:
ln A = 15.0
A = e^15.0
A = 3.27 * 10^6
E/R = 106/T
E = R * (106/T)
E = (8.314 J/mol*K) * (106/T)
E = 8.314 * 10^-3 * (10^6/T)
E = 8.314 * 10^3 / T
Substituting the value of T = 300 K (room temperature), we get:
E = 8.314 * 10^3 / 300
E = 27.71 kJ/mol
E = 2.77 * 10^4 J/mol
Therefore, the correct pair of values for A and E is:
A = 3.27 * 10^6
E = 2.77 * 10^4 J/mol
Hence, option 'A' is the correct answer.
The first-order rate constant k is related to temperature as log k = 15.0 - (106/T)
To find:
The correct pair of values for A and E.
Solution:
The Arrhenius equation is given by:
k = A * e^(-E/RT)
where,
k = rate constant
A = pre-exponential factor
E = activation energy
R = universal gas constant = 8.314 J/mol*K
T = absolute temperature
Taking natural logarithm on both sides of the equation, we get:
ln k = ln A - (E/RT)
Comparing this equation with the given equation, we can see that:
ln A = 15.0
E/R = 106/T
Solving for A and E, we get:
ln A = 15.0
A = e^15.0
A = 3.27 * 10^6
E/R = 106/T
E = R * (106/T)
E = (8.314 J/mol*K) * (106/T)
E = 8.314 * 10^-3 * (10^6/T)
E = 8.314 * 10^3 / T
Substituting the value of T = 300 K (room temperature), we get:
E = 8.314 * 10^3 / 300
E = 27.71 kJ/mol
E = 2.77 * 10^4 J/mol
Therefore, the correct pair of values for A and E is:
A = 3.27 * 10^6
E = 2.77 * 10^4 J/mol
Hence, option 'A' is the correct answer.
The rate constant, the activation energy and the frequency factor of a chemical reaction at 25°C are 3.0 × 10-2 s-1, 104.4 KJ mol-1 and 6.0 × 1014 s-1 respectively. The value of the rate constant as T → ∞ is :- a)2.0 × 1018 s-1
- b)6.0 × 1014 s-1
- c)Infinity
- d)3.6 × 1030 s-1
Correct answer is option 'B'. Can you explain this answer?
The rate constant, the activation energy and the frequency factor of a chemical reaction at 25°C are 3.0 × 10-2 s-1, 104.4 KJ mol-1 and 6.0 × 1014 s-1 respectively. The value of the rate constant as T → ∞ is :
a)
2.0 × 1018 s-1
b)
6.0 × 1014 s-1
c)
Infinity
d)
3.6 × 1030 s-1
|
|
Om Desai answered |
K = 3 × 10–2 s–1
Ea = 104.4 KJ/mol
A = 6 × 1014
Value of rate constant at T = ∞ will be equal to frequency factor i.e. A = 6 × 1014 s-1
Ea = 104.4 KJ/mol
A = 6 × 1014
Value of rate constant at T = ∞ will be equal to frequency factor i.e. A = 6 × 1014 s-1
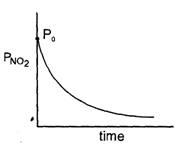
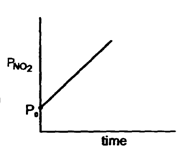
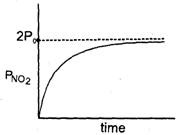
N205 ---* 2NO2 + O2When N205 decompose, its t12 does not change with its changing pressure during the reaction, so which one is the correct representation for "pressure of 2NO2" vs lime° during the reaction when initial N205 is equals to Po- a)
- b)
- c)
- d)
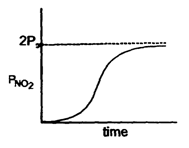
Correct answer is option 'C'. Can you explain this answer?
N205 ---* 2NO2 + O2
When N205 decompose, its t12 does not change with its changing pressure during the reaction, so which one is the correct representation for "pressure of 2NO2" vs lime° during the reaction when initial N205 is equals to Po
a)
b)
c)
d)
|
|
Nandini Iyer answered |
The correct sentence is option C
Since the half-life period is independent of the changing pressure, the reaction is of the first order.
The integrated rate law is (PN2O5)t=(PoN2O5)0e−kt
Thus the partial pressure of N2O5 decreases exponentially with time and the partial pressure of NO2 increases exponentially with time.
Hence, the correct graph is represented by option C.
Since the half-life period is independent of the changing pressure, the reaction is of the first order.
The integrated rate law is (PN2O5)t=(PoN2O5)0e−kt
Thus the partial pressure of N2O5 decreases exponentially with time and the partial pressure of NO2 increases exponentially with time.
Hence, the correct graph is represented by option C.
For the first order reaction A → B + C, carried out at 27°C if 3.8 × 10-16% of the reactant molecules exists in the activated state, the Ea (activation energy) of the reaction is- a)12 kJ/mole
- b)831.4 kJ/mole
- c)100 KJ/mole
- d)88.57 kJ/mole
Correct answer is option 'C'. Can you explain this answer?
For the first order reaction A → B + C, carried out at 27°C if 3.8 × 10-16% of the reactant molecules exists in the activated state, the Ea (activation energy) of the reaction is
a)
12 kJ/mole
b)
831.4 kJ/mole
c)
100 KJ/mole
d)
88.57 kJ/mole
|
|
Lavanya Menon answered |
A → B + C
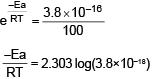
Ea = 100 KJ/mol
Ea = 100 KJ/mol
Radioactivity of a sample (z = 22) decreases 90% after 10 years. What will be the half-life of the sample?- a)5 years
- b)2 years
- c)3 years
- d)10 years
Correct answer is option 'C'. Can you explain this answer?
Radioactivity of a sample (z = 22) decreases 90% after 10 years. What will be the half-life of the sample?
a)
5 years
b)
2 years
c)
3 years
d)
10 years
|
|
Maulik Dasgupta answered |
Half-life is defined as the time it takes for half of the radioactive nuclei in a sample to decay. In this case, we are given that the radioactivity of a sample with atomic number (z) 22 decreases by 90% after 10 years. We need to determine the half-life of the sample.
Let's assume that initially, the sample had N0 radioactive nuclei. After 10 years, the radioactivity of the sample decreases by 90%, which means only 10% of the radioactive nuclei remain.
Therefore, the number of radioactive nuclei remaining after 10 years is given by:
N10 = 0.10 * N0
We can express the ratio of the number of radioactive nuclei remaining after a certain time (Nt) to the initial number of radioactive nuclei (N0) as:
Nt/N0 = (1/2)^(t/T)
Where T is the half-life of the sample and t is the time.
In this case, we can substitute N10 = 0.10 * N0 and t = 10 years into the equation to solve for T:
N10/N0 = (1/2)^(10/T)
0.10 = (1/2)^(10/T)
Taking the logarithm of both sides:
log(0.10) = log((1/2)^(10/T))
log(0.10) = (10/T) * log(1/2)
Using the property log(a^b) = b * log(a), we can rewrite the equation as:
log(0.10) = (10/T) * (-log(2))
Now we can solve for T by rearranging the equation:
T = (10 * log(2)) / log(0.10)
Using a calculator, we find that T ≈ 3.32 years.
Since we are looking for the half-life in years, the closest option is c) 3 years.
Let's assume that initially, the sample had N0 radioactive nuclei. After 10 years, the radioactivity of the sample decreases by 90%, which means only 10% of the radioactive nuclei remain.
Therefore, the number of radioactive nuclei remaining after 10 years is given by:
N10 = 0.10 * N0
We can express the ratio of the number of radioactive nuclei remaining after a certain time (Nt) to the initial number of radioactive nuclei (N0) as:
Nt/N0 = (1/2)^(t/T)
Where T is the half-life of the sample and t is the time.
In this case, we can substitute N10 = 0.10 * N0 and t = 10 years into the equation to solve for T:
N10/N0 = (1/2)^(10/T)
0.10 = (1/2)^(10/T)
Taking the logarithm of both sides:
log(0.10) = log((1/2)^(10/T))
log(0.10) = (10/T) * log(1/2)
Using the property log(a^b) = b * log(a), we can rewrite the equation as:
log(0.10) = (10/T) * (-log(2))
Now we can solve for T by rearranging the equation:
T = (10 * log(2)) / log(0.10)
Using a calculator, we find that T ≈ 3.32 years.
Since we are looking for the half-life in years, the closest option is c) 3 years.
The activation energies of two reactions are Ea and Ea’ with Ea > Ea’ If temperature of the reacting systems is increased from T1 to T2, predict which of the following alternatives - a)
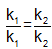
- b)
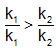
- c)
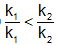
- d)
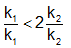
Correct answer is option 'C'. Can you explain this answer?
The activation energies of two reactions are Ea and Ea’ with Ea > Ea’ If temperature of the reacting systems is increased from T1 to T2, predict which of the following alternatives
a)
b)
c)
d)
|
|
Geetika Shah answered |
The correct answer is option B
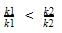
Greater the activation energy of a reaction, greater is the temperature dependence of rate constant of reaction.
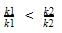
Greater the activation energy of a reaction, greater is the temperature dependence of rate constant of reaction.
For a given reaction of first order it takes 20 min. for the conc. to drop from 1.0 M to 0.60 M. The time required for the conc. to drop from 0.60 M to 0.36 M will be :- a)More than 20 min
- b)20 min
- c)Less than 30 min
- d)Cannot tell.
Correct answer is option 'B'. Can you explain this answer?
For a given reaction of first order it takes 20 min. for the conc. to drop from 1.0 M to 0.60 M. The time required for the conc. to drop from 0.60 M to 0.36 M will be :
a)
More than 20 min
b)
20 min
c)
Less than 30 min
d)
Cannot tell.
|
|
Tanvi Bose answered |
Also,
Since, Rate constant will be equal
On solving, t = 20 min
Mathematical representation for t1/4 life for first order reaction is over is given by :- a)t1/4 = [(2.303)/(K)] log 4
- b)t1/4 = [(2.303/(K)] log 3
- c)t1/4 = [(2.303)/(K)] log(4/3)
- d)t1/4 = [(2.303)/(K)]log(3/4)
Correct answer is option 'C'. Can you explain this answer?
Mathematical representation for t1/4 life for first order reaction is over is given by :
a)
t1/4 = [(2.303)/(K)] log 4
b)
t1/4 = [(2.303/(K)] log 3
c)
t1/4 = [(2.303)/(K)] log(4/3)
d)
t1/4 = [(2.303)/(K)]log(3/4)
|
|
Devanshi Mehta answered |
t1/4 = time taken for 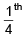 decomposition.
decomposition.
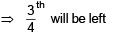
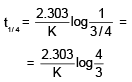
Statement-1 : If the activation energy of reaction is zero temperature will have no effect on the rate constant.Statement-2 : Lower the activation energy fasten is the reaction.- a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.
- b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1.
- c)Statement-1 is True, Statement-2 is False.
- d)Statement-1 is False, Statement-2 is True
Correct answer is option 'B'. Can you explain this answer?
Statement-1 : If the activation energy of reaction is zero temperature will have no effect on the rate constant.
Statement-2 : Lower the activation energy fasten is the reaction.
a)
Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.
b)
Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1.
c)
Statement-1 is True, Statement-2 is False.
d)
Statement-1 is False, Statement-2 is True

|
Maulik Mehra answered |
According to arrhenius equation K = AeEa/RT when Ea = 0, K = A.
The rate data for the net reaction at 25°C for the reaction X + 2Y → 3Z are given below :[X0] [Y0] Time required for [Z] to increase by 0.005 mol per litre.0.01 0.01 72 sec0.02 0.005 36 sec0.02 0.01 18 secThe intial rate (as given by Z) is :- a)First order in both X and Y
- b)Second order in X and first order in Y
- c)First order in X and second order in Y
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
The rate data for the net reaction at 25°C for the reaction X + 2Y → 3Z are given below :
[X0] [Y0] Time required for [Z] to increase by 0.005 mol per litre.
0.01 0.01 72 sec
0.02 0.005 36 sec
0.02 0.01 18 sec
The intial rate (as given by Z) is :
a)
First order in both X and Y
b)
Second order in X and first order in Y
c)
First order in X and second order in Y
d)
None of the above
|
|
Naina Datta answered |
Since,
Rate becomes four times by doubling the concentration of A
⇒ Order w.r.t A is 2
Also,
by doubling the concentration of B, the rate becomes double
⇒ Order w.r.t. B is 1.
Statement-1 : For A+ 2B → C (rate = K [A]1[B]°), the half life time of reaction is only defined when conch of A and B are in stoichometric ratioStatement-2 : For above given order half life of reaction is directly proportional to conch of A and not to conch of B due to its zero order.- a)Statement-1 is True, Statement-1 is True; Statement-1 is a correct explanation for Statement-1.
- b)Statement-1 is True, Statement-1 is True; Statement-1 is NOT a correct explanation for Statement-2.
- c)Statement-1 is True, Statement-1 is False.
- d)Statement-1 is False, Statement-1 is True
Correct answer is option 'C'. Can you explain this answer?
Statement-1 : For A+ 2B → C (rate = K [A]1[B]°), the half life time of reaction is only defined when conch of A and B are in stoichometric ratio
Statement-2 : For above given order half life of reaction is directly proportional to conch of A and not to conch of B due to its zero order.
a)
Statement-1 is True, Statement-1 is True; Statement-1 is a correct explanation for Statement-1.
b)
Statement-1 is True, Statement-1 is True; Statement-1 is NOT a correct explanation for Statement-2.
c)
Statement-1 is True, Statement-1 is False.
d)
Statement-1 is False, Statement-1 is True
|
|
Abhijeet Sharma answered |
The correct answer is option C
If the concentration of B is much lower than the stoichiometric concentration, then there will not be sufficient quantity of B to react with A and the half life period cannot be defined. Hence, the assertion is correct.
Since the reaction is first order with respect to A, the half life is independent of the concentration of A.
If the concentration of B is much lower than the stoichiometric concentration, then there will not be sufficient quantity of B to react with A and the half life period cannot be defined. Hence, the assertion is correct.
Since the reaction is first order with respect to A, the half life is independent of the concentration of A.
Units of rate constant for first and zero order reactions in terms of molarity (M) are respectively.- a)sec-1, M sec-1
- b)sec-1, M
- c)M sec-1, sec-1
- d)M, sec-1
Correct answer is option 'A'. Can you explain this answer?
Units of rate constant for first and zero order reactions in terms of molarity (M) are respectively.
a)
sec-1, M sec-1
b)
sec-1, M
c)
M sec-1, sec-1
d)
M, sec-1
|
|
Gk Bazra answered |
In first order reactions, the reaction rate is directly proportional to the reactant concentration and the units of first order rate constants are 1/sec. ... There are also zero order reactions in which the reaction is independent of the reactant concentrations where the units of the rate constant are mol/L*sec
A first order reaction is 87.5% complete in an hour. The rate constant of the reaction is- a)0.0346 min–1
- b) 0.0693 h–1
- c)0.0693 min–1
- d) 0.0346 h–1
Correct answer is option 'A'. Can you explain this answer?
A first order reaction is 87.5% complete in an hour. The rate constant of the reaction is
a)
0.0346 min–1
b)
0.0693 h–1
c)
0.0693 min–1
d)
0.0346 h–1
|
|
Aravind Rane answered |
The correct answer is Option A.
KT = ln (a0 / at)
⇒ K*60 = ln (a0 / at)
87.5 % completed in 1 hour.
Therefore, at = a0 - 0.875 a0
at = 0.125 a0
K * 60 = ln (a0 / 0.125 a0)
⇒ K * 60 = ln 8
⇒ K = 2.079 / 60
⇒ K = 0.0346 min-1
KT = ln (a0 / at)
⇒ K*60 = ln (a0 / at)
87.5 % completed in 1 hour.
Therefore, at = a0 - 0.875 a0
at = 0.125 a0
K * 60 = ln (a0 / 0.125 a0)
⇒ K * 60 = ln 8
⇒ K = 2.079 / 60
⇒ K = 0.0346 min-1
Rate law cannot be determined from balanced chemical equation if _______.- a)reverse reaction is involved.
- b) it is an elementary reaction.
- c) it is a sequence of elementary reactions.
- d)any of the reactants is in excess.
Correct answer is option 'A,C,D'. Can you explain this answer?
Rate law cannot be determined from balanced chemical equation if _______.
a)
reverse reaction is involved.
b)
it is an elementary reaction.
c)
it is a sequence of elementary reactions.
d)
any of the reactants is in excess.
|
|
Geetika Shah answered |
A catalyst is a substance that speeds up the rate of the reaction without being consumed by the reaction itself. When a catalyst is added, the activation energy is lowered because the catalyst provides a new reaction pathway with lower activation energy.
At a certain temperature, the first order rate constant k1 is found to be smaller than the second order rate constant k2. If the energy of activation E1 of the first order reaction is greater than energy of activation E2 of the second order reaction, then with increase in temperature.- a)k1 will increase faster than k2, but always will remain less than k2.
- b)k2 will increase faster than k1
- c)k1 will increase faster than k2 and becomes equal to k2.
- d)k1 will increase faster k2 and becomes greater than k2.
Correct answer is option 'A'. Can you explain this answer?
At a certain temperature, the first order rate constant k1 is found to be smaller than the second order rate constant k2. If the energy of activation E1 of the first order reaction is greater than energy of activation E2 of the second order reaction, then with increase in temperature.
a)
k1 will increase faster than k2, but always will remain less than k2.
b)
k2 will increase faster than k1
c)
k1 will increase faster than k2 and becomes equal to k2.
d)
k1 will increase faster k2 and becomes greater than k2.

|
Rahul Desai answered |
Reaction A + B → C + D follow's following rate law : rate = k[A]+1/2[B]1/2. Starting with initial conc. of 1 M of A and B each, what is the time taken for concentration of A of become 0.25 M.Given : k = 2.303 × 10-3 sec-1.- a)300 sec
- b)600 sec
- c)900 sec
- d)none of these
Correct answer is option 'B'. Can you explain this answer?
Reaction A + B → C + D follow's following rate law : rate = k[A]+1/2[B]1/2. Starting with initial conc. of 1 M of A and B each, what is the time taken for concentration of A of become 0.25 M.
Given : k = 2.303 × 10-3 sec-1.
a)
300 sec
b)
600 sec
c)
900 sec
d)
none of these
|
|
Maya Reddy answered |
Rate= K[A]1/2[B]1/2
Overall order of reaction is = 1/2+1/2=1
∴ It is a 1st order reaction.
For concentration of A to become 0.5M:-t1/2=0.693/k
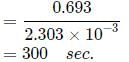
It will require 2 half times for the concentration of A to become .0.25M.
∴ Time taken= 2×t1/2 sec
=2×300
=600sec
Chemical substances speeding up rate of chemical reaction is called as- a)pressure
- b)concentration
- c)catalysts
- d)inhibitors
Correct answer is option 'C'. Can you explain this answer?
Chemical substances speeding up rate of chemical reaction is called as
a)
pressure
b)
concentration
c)
catalysts
d)
inhibitors

|
Ayush Chauhan answered |
A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; hence a catalyst can be recovered chemically unchanged at the end of the reaction it has been used to speed up, or catalyze.
Statement-1 : For a reaction A(g) → B(g) — re = 2.5 PA at 400K — re = 2.5 PA at 600Kactivation energy is 4135 J/mol.Statement-2 : Since for any reaction, values of rate constant at two different temp is same therefore activation energy of the reaction is zero.- a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.
- b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1.
- c)Statement-1 is True, Statement-2 is False.
- d)Statement-1 is False, Statement-2 is True.
Correct answer is option 'B'. Can you explain this answer?
Statement-1 : For a reaction A(g) → B(g)
— re = 2.5 PA at 400K
— re = 2.5 PA at 600K
activation energy is 4135 J/mol.
Statement-2 : Since for any reaction, values of rate constant at two different temp is same therefore activation energy of the reaction is zero.
a)
Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.
b)
Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1.
c)
Statement-1 is True, Statement-2 is False.
d)
Statement-1 is False, Statement-2 is True.

|
Ashwini Chakraborty answered |
Reaction rates are determined by all of the following factors except:- a)the number of collisions between molecules
- b)the force of collision between molecules
- c)the orientation of collision between molecules
- d)the spontaneity of the reaction
Correct answer is option 'D'. Can you explain this answer?
a)
the number of collisions between molecules
b)
the force of collision between molecules
c)
the orientation of collision between molecules
d)
the spontaneity of the reaction
|
|
Milan Dasgupta answered |
Understanding Reaction Rates
Reaction rates are influenced by several factors that determine how quickly reactants convert to products. However, the spontaneity of a reaction does not directly affect the rate at which it occurs.
Factors Affecting Reaction Rates
- Number of Collisions: The more frequently molecules collide, the higher the likelihood of reaction. Increased concentration of reactants generally leads to more collisions.
- Force of Collision: The energy with which molecules collide impacts their ability to overcome the activation energy barrier. Stronger collisions can lead to successful reactions.
- Orientation of Collision: Molecules must collide in the correct orientation for a reaction to occur. Proper alignment increases the probability of successful reactions.
Why Spontaneity Does Not Affect Reaction Rate
- Definition of Spontaneity: Spontaneity refers to whether a reaction can occur without external intervention, often indicated by a negative Gibbs free energy change (ΔG).
- Rate vs. Favorability: While a reaction may be spontaneous, it can still proceed at a very slow rate. Conversely, a non-spontaneous reaction can occur rapidly under certain conditions.
- Kinetics vs. Thermodynamics: Reaction rates (kinetics) and spontaneity (thermodynamics) are governed by different principles. Kinetics focuses on how quickly reactions occur, while thermodynamics determines the direction and feasibility of a reaction.
Conclusion
In summary, while the number, force, and orientation of collisions are critical to determining reaction rates, the spontaneity of a reaction does not influence how fast it occurs. Therefore, option 'D' is the correct answer.
Reaction rates are influenced by several factors that determine how quickly reactants convert to products. However, the spontaneity of a reaction does not directly affect the rate at which it occurs.
Factors Affecting Reaction Rates
- Number of Collisions: The more frequently molecules collide, the higher the likelihood of reaction. Increased concentration of reactants generally leads to more collisions.
- Force of Collision: The energy with which molecules collide impacts their ability to overcome the activation energy barrier. Stronger collisions can lead to successful reactions.
- Orientation of Collision: Molecules must collide in the correct orientation for a reaction to occur. Proper alignment increases the probability of successful reactions.
Why Spontaneity Does Not Affect Reaction Rate
- Definition of Spontaneity: Spontaneity refers to whether a reaction can occur without external intervention, often indicated by a negative Gibbs free energy change (ΔG).
- Rate vs. Favorability: While a reaction may be spontaneous, it can still proceed at a very slow rate. Conversely, a non-spontaneous reaction can occur rapidly under certain conditions.
- Kinetics vs. Thermodynamics: Reaction rates (kinetics) and spontaneity (thermodynamics) are governed by different principles. Kinetics focuses on how quickly reactions occur, while thermodynamics determines the direction and feasibility of a reaction.
Conclusion
In summary, while the number, force, and orientation of collisions are critical to determining reaction rates, the spontaneity of a reaction does not influence how fast it occurs. Therefore, option 'D' is the correct answer.
The inversion of cane sugar proceeds with half life of 600 minute at pH = 5 for any concentration of sugar, However at pH = 6, the half life changes to 60 minute, The rate law expression for sugar inversion can be written as- a)r = k [sugar]2 [H+]°
- b)r = k [sugar]' [Hl°
- c)r = k [sugar]2 [H+]1
- d)r = k[sugar]°[H+]-1
Correct answer is option 'B'. Can you explain this answer?
The inversion of cane sugar proceeds with half life of 600 minute at pH = 5 for any concentration of sugar, However at pH = 6, the half life changes to 60 minute, The rate law expression for sugar inversion can be written as
a)
r = k [sugar]2 [H+]°
b)
r = k [sugar]' [Hl°
c)
r = k [sugar]2 [H+]1
d)
r = k[sugar]°[H+]-1

|
Dishani Kulkarni answered |
The reaction 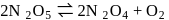 is
is- a)bimolecular and of second order
- b)unimolecular and of first order
- c)bimolecular and of first order
- d)bimolecular and of zero order
Correct answer is option 'C'. Can you explain this answer?
The reaction 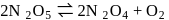 is
is
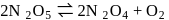 is
isa)
bimolecular and of second order
b)
unimolecular and of first order
c)
bimolecular and of first order
d)
bimolecular and of zero order

|
EduRev JEE answered |
It is bimolecular first order reaction since Rate 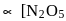 ]
]
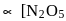 ]
]For a reaction A → Products, the conc. of reactant C0, aC0, a2C0, a3C0............ after time interval 0, t, 2t ............ where 'a' is constant. Then :- a)Reaction is of 1 st order and K = (1/t) ln a
- b)Reaction is of 2nd order and K = (1/tC0)(1 – a)/a
- c)Reaction is of 1st order and K = (1/t) ln (1/a)
- d)Reaction is of zero order and K = {(1 – a)}C0/t
Correct answer is option 'C'. Can you explain this answer?
For a reaction A → Products, the conc. of reactant C0, aC0, a2C0, a3C0............ after time interval 0, t, 2t ............ where 'a' is constant. Then :
a)
Reaction is of 1 st order and K = (1/t) ln a
b)
Reaction is of 2nd order and K = (1/tC0)(1 – a)/a
c)
Reaction is of 1st order and K = (1/t) ln (1/a)
d)
Reaction is of zero order and K = {(1 – a)}C0/t
|
|
Rhea Iyer answered |
If we calculate K, it comes constant every time i.e. reaction is of first order.
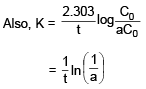
Instantaneous reaction expresses- a)Rate at particular moment of time
- b)Pressure at particular time
- c)Speed at particular time
- d)Concentration at particular time
Correct answer is option 'A'. Can you explain this answer?
Instantaneous reaction expresses
a)
Rate at particular moment of time
b)
Pressure at particular time
c)
Speed at particular time
d)
Concentration at particular time

|
Ayush Chauhan answered |
The initial rate of a reaction is the instantaneous rate at the start of the reaction (i.e., when t = 0). The initial rate is equal to the negative of the slope of the curve of reactant concentration versus time at t = 0.
Compounds A and B react with a common reagent with first order kinetics in both cases. If 99% of A must react before 1% of B has reacted. What is the minimum ratio for their respective rate constants? - a)916
- b)229
- c)500
- d)458
Correct answer is option 'D'. Can you explain this answer?
Compounds A and B react with a common reagent with first order kinetics in both cases. If 99% of A must react before 1% of B has reacted. What is the minimum ratio for their respective rate constants?
a)
916
b)
229
c)
500
d)
458

|
Sankar Gupta answered |
Consider the following reaction
A⟶ Products
This reaction is completed in 100 min. The rate constant of this reaction at t1 = 10 min, is 10−2 min−1. What is the rate constant (in min−1) at t2 = 20 min?- a)
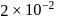
- b)
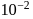
- c)
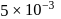
- d)
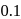
Correct answer is option 'B'. Can you explain this answer?
Consider the following reaction
A⟶ Products
This reaction is completed in 100 min. The rate constant of this reaction at t1 = 10 min, is 10−2 min−1. What is the rate constant (in min−1) at t2 = 20 min?
A⟶ Products
This reaction is completed in 100 min. The rate constant of this reaction at t1 = 10 min, is 10−2 min−1. What is the rate constant (in min−1) at t2 = 20 min?
a)
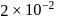
b)
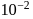
c)
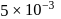
d)
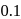

|
Manish Aggarwal answered |
For a first order reaction, rate constant does not depend upon concentration.
∴ Rate constant at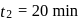 also
also 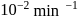 (constant).
(constant).
∴ Rate constant at
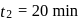 also
also 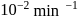 (constant).
(constant).In the reaction : A + 2B → 3C + D, which of the following expression does not describe changes in the conc. of various species as a function of time :- a){-d[C]/dt} = {3/2d[B]/dt}
- b){3d[D]/dt} = {d[C]/dt}
- c){3d[B]/dt} = {-2d[C]/dt}
- d){2d[B]/dt} = {d[A]/dt}
Correct answer is option 'D'. Can you explain this answer?
In the reaction : A + 2B → 3C + D, which of the following expression does not describe changes in the conc. of various species as a function of time :
a)
{-d[C]/dt} = {3/2d[B]/dt}
b)
{3d[D]/dt} = {d[C]/dt}
c)
{3d[B]/dt} = {-2d[C]/dt}
d)
{2d[B]/dt} = {d[A]/dt}

|
Ishita Deshpande answered |
Chapter doubts & questions for Chemical Kinetics - Chemistry for JEE Main & Advanced 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Kinetics - Chemistry for JEE Main & Advanced in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup