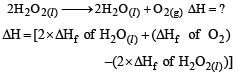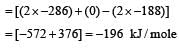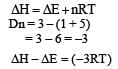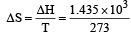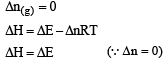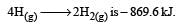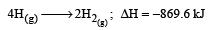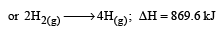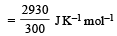All Exams >
NEET >
NEET Past Year Papers >
All Questions
All questions of Thermodynamics for NEET Exam
A reaction occurs spontaneously if [2005]a) TΔS < ΔH and both ΔH and ΔS are + veb) TΔS > ΔH an d ΔH is + ve and ΔS is -vec) TΔS > ΔH and both ΔH and ΔS are + ved) TΔS = ΔH and both ΔH and ΔS are + veCorrect answer is option 'C'. Can you explain this answer?

|
Abhishek Choudhary answered |
For a spontaneous reaction ΔG(–ve), which is possible if ΔS = +ve, ΔH = +ve and TΔS > ΔH [As ΔG = ΔH – TΔS]
Heat of combustion ΔHº for C (s), H2(g) and CH4(g) are –94, –68 and –213 kcal/mol, then ΔHº for C(s) + 2H2(g) → CH4(g) is [2002]- a)–17 kcal
- b)– 111 kcal
- c)–170 kcal
- d)–85 kcal
Correct answer is option 'A'. Can you explain this answer?
Heat of combustion ΔHº for C (s), H2(g) and CH4(g) are –94, –68 and –213 kcal/mol, then ΔHº for C(s) + 2H2(g) → CH4(g) is [2002]
a)
–17 kcal
b)
– 111 kcal
c)
–170 kcal
d)
–85 kcal

|
Savita Soni answered |
∆Hcomb.= Reactant - product
so -94-68×2(because there are 2moles of H2)-(-213)
∆Hcomb.= -17 kcal
so -94-68×2(because there are 2moles of H2)-(-213)
∆Hcomb.= -17 kcal
A chemical reaction will be spontaneous if it is accompanied by a decrease of [1994]- a)entropy of the system
- b)enthalpy of the system
- c)internal energy of the system
- d)free energy of the system
Correct answer is option 'D'. Can you explain this answer?
A chemical reaction will be spontaneous if it is accompanied by a decrease of [1994]
a)
entropy of the system
b)
enthalpy of the system
c)
internal energy of the system
d)
free energy of the system

|
Raghav Khanna answered |
ΔG is negative for a spontaneous process.
Bond dissociation enthalpy of H2, Cl2 and HCl are 434 , 242 and 431 kJ mol–1 respectively.Enthalpy of formation of HCl is : [2008]- a)93 kJ mol–1
- b)– 245 kJmol–1
- c)– 93 kJmol–1
- d)245 kJmol–1
Correct answer is option 'C'. Can you explain this answer?
Bond dissociation enthalpy of H2, Cl2 and HCl are 434 , 242 and 431 kJ mol–1 respectively.Enthalpy of formation of HCl is : [2008]
a)
93 kJ mol–1
b)
– 245 kJmol–1
c)
– 93 kJmol–1
d)
245 kJmol–1

|
Rohan Unni answered |
The reaction for formation of HCl can be written as H2 + Cl2 → 2HCI
H – H + Cl – Cl → 2 (H – Cl)
Substituting the given values, we get enthalpy of formation of 2HCl = – ( 862 – 676) = –186 kJ.
∴ Enthalpy of formation of
H – H + Cl – Cl → 2 (H – Cl)
Substituting the given values, we get enthalpy of formation of 2HCl = – ( 862 – 676) = –186 kJ.
∴ Enthalpy of formation of
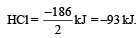
The absolute enthalpy of neutralisation of the reaction: MgO (s) + 2HCl (aq) —→ MgCl2(aq) + H2O (l) will be:[2005]- a)-57.33 kJ mol-1
- b)Greater than -57.33 kJ mol-1
- c)Less than -57.33 kJ mol-1
- d)57.33 kJ mol-1
Correct answer is option 'C'. Can you explain this answer?
The absolute enthalpy of neutralisation of the reaction: MgO (s) + 2HCl (aq) —→ MgCl2(aq) + H2O (l) will be:[2005]
a)
-57.33 kJ mol-1
b)
Greater than -57.33 kJ mol-1
c)
Less than -57.33 kJ mol-1
d)
57.33 kJ mol-1

|
Swara Desai answered |
As MgO is a oxide of weak base hence some energy is lost to break MgO (s). Hence enthalpy is less than –57.33 kJ mol–1.
Which of the following pairs of a chemical reaction is certain to result in a spontaneous reaction? [2005]- a)Exothermic and increasing disorder
- b)Exothermic and decreasing disorder
- c)Endothermic and increasing disorder
- d)Endothermic and decreasing disorder
Correct answer is option 'A'. Can you explain this answer?
Which of the following pairs of a chemical reaction is certain to result in a spontaneous reaction? [2005]
a)
Exothermic and increasing disorder
b)
Exothermic and decreasing disorder
c)
Endothermic and increasing disorder
d)
Endothermic and decreasing disorder

|
Arpita Tiwari answered |
Measure of disor der of a system is noth ing but Entropy. For a spontaneous reaction, ΔG < 0. As per Gibbs Helmnoltz equation, ΔG = ΔH – TΔS
Thus ΔG is –ve only When ΔH = –ve (exothermic) and ΔS = +ve (increasing disorder)
Thus ΔG is –ve only When ΔH = –ve (exothermic) and ΔS = +ve (increasing disorder)
During isothermal expansion of an ideal gas, its- a)internal energy increases [1991, 94]
- b)enthalpy decreases
- c)enthalpy remains unaffected
- d)enthalpy reduces to zero.
Correct answer is option 'C'. Can you explain this answer?
During isothermal expansion of an ideal gas, its
a)
internal energy increases [1991, 94]
b)
enthalpy decreases
c)
enthalpy remains unaffected
d)
enthalpy reduces to zero.

|
Shanaya Rane answered |
During isothermal expansion of ideal gas,
ΔT = 0. Now H = E + PV
∵ ΔH = ΔE + Δ(PV)
∴ ΔH = ΔE + Δ(nRTT));
Thus if ΔT = 0., ΔH = ΔE i.e., remain unaffected
ΔT = 0. Now H = E + PV
∵ ΔH = ΔE + Δ(PV)
∴ ΔH = ΔE + Δ(nRTT));
Thus if ΔT = 0., ΔH = ΔE i.e., remain unaffected
Adiabatice xpansions of an ideal gas is accompanied by [1999]- a)decrease in ΔE
- b)increase in temperature
- c)decrease in ΔS
- d)no change in anyone of the a bove properties
Correct answer is option 'A'. Can you explain this answer?
Adiabatice xpansions of an ideal gas is accompanied by [1999]
a)
decrease in ΔE
b)
increase in temperature
c)
decrease in ΔS
d)
no change in anyone of the a bove properties

|
Arya Khanna answered |
ΔE = ΔQ–W
For adiabatic expansion,ΔQ = 0
⇒ ΔE = –W
The negative sign shows decrease in Internal energy, which is equal to the work done on the system by the surroundings.
For adiabatic expansion,ΔQ = 0
⇒ ΔE = –W
The negative sign shows decrease in Internal energy, which is equal to the work done on the system by the surroundings.
In which of the following reactions, standard entropy change (ΔS°) is positive and standard Gibb’s energy change (ΔG°) decreases sharply with increasing temperature ? [2012]- a)C graphite
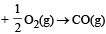
- b)
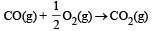
- c)
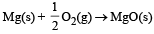
- d)
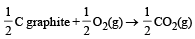
Correct answer is option 'A'. Can you explain this answer?
In which of the following reactions, standard entropy change (ΔS°) is positive and standard Gibb’s energy change (ΔG°) decreases sharply with increasing temperature ? [2012]
a)
C graphite 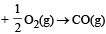
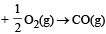
b)
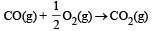
c)
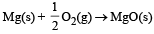
d)
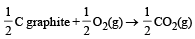

|
Devansh Mehra answered |
Since, in the first reaction gaseous products are forming from solid carbon hence entropy will increase i.e. Δs = +ve.
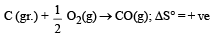
Since, ΔG° = ΔH° – TΔS hence the value of ΔG decrease on increasing temperature.
For the gas phase reaction, [2008] which of the following conditions are correct ?- a)ΔH = 0 and ΔS < 0
- b)ΔH > 0 and ΔS > 0
- c)ΔH < 0 and ΔS < 0
- d)ΔH > 0 and ΔS < 0
Correct answer is option 'B'. Can you explain this answer?
For the gas phase reaction, [2008] which of the following conditions are correct ?
a)
ΔH = 0 and ΔS < 0
b)
ΔH > 0 and ΔS > 0
c)
ΔH < 0 and ΔS < 0
d)
ΔH > 0 and ΔS < 0

|
Arpita Tiwari answered |
For the reaction
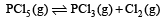
The reaction given is an example of decomposition reaction and we know that decomposition reactions are endothermic in nature, i.e, ΔH > 0.
Further Δn = (1+1) – 1= +1 Hence more number of molecules are present in products which shows more randomness i.e. ΔS > 0 (ΔS is positive)
Further Δn = (1+1) – 1= +1 Hence more number of molecules are present in products which shows more randomness i.e. ΔS > 0 (ΔS is positive)
The values of ΔH and ΔS for the reaction, C(graphite) + CO2 (g) → 2CO(g) are 170 kJ and 170 JK–1, respectively. This reaction will be spontaneous at [2009]- a)910 K
- b)1110 K
- c)510 K
- d)710 K
Correct answer is option 'B'. Can you explain this answer?
The values of ΔH and ΔS for the reaction, C(graphite) + CO2 (g) → 2CO(g) are 170 kJ and 170 JK–1, respectively. This reaction will be spontaneous at [2009]
a)
910 K
b)
1110 K
c)
510 K
d)
710 K

|
Lekshmi Banerjee answered |
ΔG = ΔH – T Δ S At equilibrium, ΔG = 0
⇒ 0 = (170 × 103J) – T (170JK– 1)
⇒ T = 1000 K
For spontaneity, ΔG is – ve, which is possible only if T > 1000 K.
⇒ 0 = (170 × 103J) – T (170JK– 1)
⇒ T = 1000 K
For spontaneity, ΔG is – ve, which is possible only if T > 1000 K.
For a cyclic process, which of the following is not true? [1999]- a)ΔH = 0
- b)ΔE = 0
- c)ΔG = 0
- d)Total W = 0
Correct answer is option 'D'. Can you explain this answer?
For a cyclic process, which of the following is not true? [1999]
a)
ΔH = 0
b)
ΔE = 0
c)
ΔG = 0
d)
Total W = 0

|
Muskaan Basak answered |
For a cyclic process
ΔE = 0, ΔH = 0 & ΔG = 0 .
As all depend upon final state and initial state,w doesn’t depend on path followed.
2 mole of an ideal gas at 27ºC temperature is expanded reversibly from 2 lit to 20 lit. Find the entropy change (R = 2 cal/mol K) [2002]- a)92.1
- b)0
- c)4
- d)9.2
Correct answer is option 'D'. Can you explain this answer?
2 mole of an ideal gas at 27ºC temperature is expanded reversibly from 2 lit to 20 lit. Find the entropy change (R = 2 cal/mol K) [2002]
a)
92.1
b)
0
c)
4
d)
9.2

|
Ashwini Khanna answered |
For isothermal reversible expansion


Entropy change, 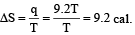
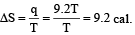
One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The ΔE for this process is (R =2 cal. mol–1 K–1)[1998]- a)163.7 cal
- b)zero
- c)1381.1 cal
- d)9 lit. atm
Correct answer is option 'B'. Can you explain this answer?
One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The ΔE for this process is (R =2 cal. mol–1 K–1)[1998]
a)
163.7 cal
b)
zero
c)
1381.1 cal
d)
9 lit. atm

|
Srishti Sen answered |
For an isother mal process ΔE = 0
When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,
ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]- a)32
- b)38
- c)317
- d)477
Correct answer is option 'C'. Can you explain this answer?
When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,
ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]
ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]
a)
32
b)
38
c)
317
d)
477

|
Pankaj Banerjee answered |
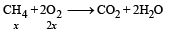
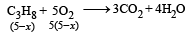
2x + 5(5– x) = 16
⇒ x = 3L
∴ Heat released
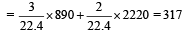
Given the following entropy values (in J K–1 mol–1) at 298 K and 1 atm :H2(g) : 130.6, Cl2(g) : 223.0, HCl (g) : 186.7.The entropy change (in J K–1 mol– 1) for the reaction H2(g) + Cl 2(g) —→ 2 HCl(g) is [1996]- a)+540.3
- b)+727.0
- c)–166.9
- d)+19.8
Correct answer is option 'D'. Can you explain this answer?
Given the following entropy values (in J K–1 mol–1) at 298 K and 1 atm :H2(g) : 130.6, Cl2(g) : 223.0, HCl (g) : 186.7.The entropy change (in J K–1 mol– 1) for the reaction H2(g) + Cl 2(g) —→ 2 HCl(g) is [1996]
a)
+540.3
b)
+727.0
c)
–166.9
d)
+19.8

|
Raksha Iyer answered |
Understanding Entropy Change
The entropy change (ΔS) for a chemical reaction can be calculated using the standard entropy values of the reactants and products.
Given Data
- Standard Entropy of H2(g): 130.6 J K–1 mol–1
- Standard Entropy of Cl2(g): 223.0 J K–1 mol–1
- Standard Entropy of HCl(g): 186.7 J K–1 mol–1
Reaction
The reaction we are considering is:
H2(g) + Cl2(g) → 2 HCl(g)
Calculating Entropy Change
The formula for calculating the entropy change for the reaction is:
ΔS = ΣS(products) - ΣS(reactants)
Step-by-Step Calculation
- Entropy of Products:
- 2 moles of HCl: 2 × 186.7 J K–1 mol–1 = 373.4 J K–1 mol–1
- Entropy of Reactants:
- H2: 130.6 J K–1 mol–1
- Cl2: 223.0 J K–1 mol–1
- Total for Reactants = 130.6 + 223.0 = 353.6 J K–1 mol–1
Final Calculation
- ΔS = 373.4 J K–1 mol–1 - 353.6 J K–1 mol–1
- ΔS = 19.8 J K–1 mol–1
Conclusion
The entropy change for the reaction H2(g) + Cl2(g) → 2 HCl(g) is 19.8 J K–1 mol–1, confirming that option 'D' is the correct answer. This indicates that the formation of HCl from H2 and Cl2 results in a slight increase in disorder, represented by a positive change in entropy.
The entropy change (ΔS) for a chemical reaction can be calculated using the standard entropy values of the reactants and products.
Given Data
- Standard Entropy of H2(g): 130.6 J K–1 mol–1
- Standard Entropy of Cl2(g): 223.0 J K–1 mol–1
- Standard Entropy of HCl(g): 186.7 J K–1 mol–1
Reaction
The reaction we are considering is:
H2(g) + Cl2(g) → 2 HCl(g)
Calculating Entropy Change
The formula for calculating the entropy change for the reaction is:
ΔS = ΣS(products) - ΣS(reactants)
Step-by-Step Calculation
- Entropy of Products:
- 2 moles of HCl: 2 × 186.7 J K–1 mol–1 = 373.4 J K–1 mol–1
- Entropy of Reactants:
- H2: 130.6 J K–1 mol–1
- Cl2: 223.0 J K–1 mol–1
- Total for Reactants = 130.6 + 223.0 = 353.6 J K–1 mol–1
Final Calculation
- ΔS = 373.4 J K–1 mol–1 - 353.6 J K–1 mol–1
- ΔS = 19.8 J K–1 mol–1
Conclusion
The entropy change for the reaction H2(g) + Cl2(g) → 2 HCl(g) is 19.8 J K–1 mol–1, confirming that option 'D' is the correct answer. This indicates that the formation of HCl from H2 and Cl2 results in a slight increase in disorder, represented by a positive change in entropy.
Which of the following are not state functions ?
(I) q + w
(II) q [2008]
(II) w
(IV) H - TS- a)(I) and (IV)
- b)(II), (III) and (IV)
- c)(I), (II) and (III)
- d)(II) and (III)
Correct answer is option 'D'. Can you explain this answer?
Which of the following are not state functions ?
(I) q + w
(II) q [2008]
(II) w
(IV) H - TS
(I) q + w
(II) q [2008]
(II) w
(IV) H - TS
a)
(I) and (IV)
b)
(II), (III) and (IV)
c)
(I), (II) and (III)
d)
(II) and (III)

|
Shruti Chauhan answered |
We know that q (heat and work (w) are not state functions but (q + w) is a state functions. H – TS (i.e. G) is also a state functions. Thus II and III are not state functions so the correct answer is option (d).
If ΔH is the change in enthalpy and ΔE, the change in internal energy accompanying a gaseous reaction, then [1990]- a)ΔH is always greater than ΔE,
- b)ΔH < Δ E only if the number of moles of the products is greater than the number of moles of the reactants
- c)Δ H is always less than Δ E
- d)Δ H < Δ E only if the number of moles of products is less than the number of moles of the reactants.
Correct answer is option 'D'. Can you explain this answer?
If ΔH is the change in enthalpy and ΔE, the change in internal energy accompanying a gaseous reaction, then [1990]
a)
ΔH is always greater than ΔE,
b)
ΔH < Δ E only if the number of moles of the products is greater than the number of moles of the reactants
c)
Δ H is always less than Δ E
d)
Δ H < Δ E only if the number of moles of products is less than the number of moles of the reactants.
|
|
Shivani Joshi answered |
You could have any superpower, what would it be and why?
For a hypothetical reaction A → B , the activation energies for forward and backward reactions are 19 kJ/mole and 9 kJ/mole respectively. Th e heat of reaction is [2000]- a)28 kJ
- b)19 kJ
- c)– 10 kJ
- d)9 kJ
Correct answer is option 'C'. Can you explain this answer?
For a hypothetical reaction A → B , the activation energies for forward and backward reactions are 19 kJ/mole and 9 kJ/mole respectively. Th e heat of reaction is [2000]
a)
28 kJ
b)
19 kJ
c)
– 10 kJ
d)
9 kJ

|
Shruti Chauhan answered |
Heat of reaction ΔH = Ebackward - Eforward
= 9 – 19 = –10 kJ/mol
= 9 – 19 = –10 kJ/mol
If the bond energies of H - H, Br - Br, and HBr are 433, 192 and 364 kJ mol–1 respectively, the ΔH° for the reaction H2(g) + Br2(g) → 2HBr(g) is [2004] - a)– 261 kJ
- b)+ 103 kJ
- c)+ 261kJ
- d)– 103 kJ
Correct answer is option 'D'. Can you explain this answer?
If the bond energies of H - H, Br - Br, and HBr are 433, 192 and 364 kJ mol–1 respectively, the ΔH° for the reaction H2(g) + Br2(g) → 2HBr(g) is [2004]
a)
– 261 kJ
b)
+ 103 kJ
c)
+ 261kJ
d)
– 103 kJ

|
Priyanka Iyer answered |
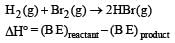
= (433 + 192) - (2x364)
= 625 – 728 = – 103 kJ
= 625 – 728 = – 103 kJ
If enthalpies of formation of C2H4(g) , CO2(g) and H2O(l) at 25°C and 1atm pressure are 52, – 394 and – 286 kJ/mol respectively, the change in ethalpy is equal to [1995]- a)– 141.2 kJ/mol
- b)– 1412 kJ/mol
- c)+ 14.2 kJ/mol
- d)+ 1412 kJ/mol
Correct answer is option 'B'. Can you explain this answer?
If enthalpies of formation of C2H4(g) , CO2(g) and H2O(l) at 25°C and 1atm pressure are 52, – 394 and – 286 kJ/mol respectively, the change in ethalpy is equal to [1995]
a)
– 141.2 kJ/mol
b)
– 1412 kJ/mol
c)
+ 14.2 kJ/mol
d)
+ 1412 kJ/mol

|
Rhea Sarkar answered |
Enthalpy of formation of C2H4 , CO2 and H2O are 52 , – 394 and – 2 86 k J/ mol respectively. (Given) The reaction is
C2H4 + 3O2 → 2CO2 + 2H2O.
change in enthalpy, (ΔH) = ΔHproducts - ΔHreactants
= 2 x (-394) + 2 x (-286) - (52 + 0)
= – 1412 kJ/ mol.
C2H4 + 3O2 → 2CO2 + 2H2O.
change in enthalpy, (ΔH) = ΔHproducts - ΔHreactants
= 2 x (-394) + 2 x (-286) - (52 + 0)
= – 1412 kJ/ mol.
The molar heat capacity of water at constant pressure is 75 JK–1 mol–1. When 1kJ of heat is supplied to 100 g of water, which is free to expand, the increase in temperature of water is [2003]- a)6.6 K
- b)1.2 K
- c)2.4 K
- d)4.8 K
Correct answer is option 'C'. Can you explain this answer?
The molar heat capacity of water at constant pressure is 75 JK–1 mol–1. When 1kJ of heat is supplied to 100 g of water, which is free to expand, the increase in temperature of water is [2003]
a)
6.6 K
b)
1.2 K
c)
2.4 K
d)
4.8 K
|
|
Pranav Datta answered |
Per mol K.
The work don e dur ing the expansion of a gas from a volume of 4 dm3 to 6 dm3 against a constant external pressure of 3 atm is (1L atm = 101.32J) [2004]- a)– 6J
- b)– 608J
- c)+ 304J
- d)– 304J
Correct answer is option 'B'. Can you explain this answer?
The work don e dur ing the expansion of a gas from a volume of 4 dm3 to 6 dm3 against a constant external pressure of 3 atm is (1L atm = 101.32J) [2004]
a)
– 6J
b)
– 608J
c)
+ 304J
d)
– 304J

|
Sounak Chaudhary answered |
The work done during the expansion of a gas can be calculated using the formula:
Work = Pressure * Change in Volume
In this case, the pressure is constant at 3 atm and the change in volume is from 4 dm3 to 6 dm3.
Change in Volume = Final Volume - Initial Volume
= 6 dm3 - 4 dm3
= 2 dm3
Plugging these values into the formula, we get:
Work = 3 atm * 2 dm3
Since 1 dm3 = 1 L, we can convert dm3 to L:
Work = 3 atm * 2 L
Finally, we can convert atm L to J using the conversion factor 1 L atm = 101.32 J:
Work = 3 * 2 * 101.32 J
= 606.72 J
Therefore, the work done during the expansion of the gas is 606.72 J.
Work = Pressure * Change in Volume
In this case, the pressure is constant at 3 atm and the change in volume is from 4 dm3 to 6 dm3.
Change in Volume = Final Volume - Initial Volume
= 6 dm3 - 4 dm3
= 2 dm3
Plugging these values into the formula, we get:
Work = 3 atm * 2 dm3
Since 1 dm3 = 1 L, we can convert dm3 to L:
Work = 3 atm * 2 L
Finally, we can convert atm L to J using the conversion factor 1 L atm = 101.32 J:
Work = 3 * 2 * 101.32 J
= 606.72 J
Therefore, the work done during the expansion of the gas is 606.72 J.
Identify the correct statement for change of Gibbs energy for a system (ΔGsystem) at constant temperature and pressure : [2006]- a)If ΔGsystem = 0, the system has attain ed equilibrium
- b)If ΔGsystem = 0, the system is still moving in a particular direction
- c)If ΔGsystem < 0 , the process is not spontaneous
- d)If ΔGsystem >0 , the process is not spontaneous
Correct answer is option 'A'. Can you explain this answer?
Identify the correct statement for change of Gibbs energy for a system (ΔGsystem) at constant temperature and pressure : [2006]
a)
If ΔGsystem = 0, the system has attain ed equilibrium
b)
If ΔGsystem = 0, the system is still moving in a particular direction
c)
If ΔGsystem < 0 , the process is not spontaneous
d)
If ΔGsystem >0 , the process is not spontaneous

|
Srishti Sen answered |
If ΔGsystem = 0 the system has attained equilibrium is right choice.
In it alternative (d) is most confusing as when ΔG > 0, the process may be spontaneous when it is coupled with a reaction which has ΔG < 0 and total ΔG is negative, so right answer is (a).
In it alternative (d) is most confusing as when ΔG > 0, the process may be spontaneous when it is coupled with a reaction which has ΔG < 0 and total ΔG is negative, so right answer is (a).
The enthalpy of hydrogenation of cyclohexane is – 119.5 kJ mol–1. If resonance energy of benzene is –150.4 kJ mol–1, its enthalpy of hydrogenation would be [2006]- a)– 208.1 kg mol–1
- b)– 269.9 kg mol–1
- c)– 358.5 kg mol–1
- d)– 508.9 kg mol–1
Correct answer is option 'A'. Can you explain this answer?
The enthalpy of hydrogenation of cyclohexane is – 119.5 kJ mol–1. If resonance energy of benzene is –150.4 kJ mol–1, its enthalpy of hydrogenation would be [2006]
a)
– 208.1 kg mol–1
b)
– 269.9 kg mol–1
c)
– 358.5 kg mol–1
d)
– 508.9 kg mol–1
|
|
Anjali Iyer answered |
Cyclohexene after hydrogenation would be converted to purely saturated cyclohexane.
As cyclohexene has one pi bond its enthalpy of hydrogenation is -119.5 kJ mol-1.
In benzene there are 3 pi bonds hence the enthalpy of hydrogenation = -150.4 kJ – 3 * -119.5 kJ.
– 208.1 kg mol–1
Hydrogen has an ionisation energy of 1311 kJ mol–1 and for chlorine it is 1256 kJ mol–1.Hydrogen forms H+ (aq) ions but chlorine does not form Cl+ (aq) ions because [1996]- a)H+ has lower hydration enthalpy
- b)Cl+ has lower hydration enthalpy
- c)Cl has high electron a ffinity
- d)Cl has high electron egativity
Correct answer is option 'B'. Can you explain this answer?
Hydrogen has an ionisation energy of 1311 kJ mol–1 and for chlorine it is 1256 kJ mol–1.Hydrogen forms H+ (aq) ions but chlorine does not form Cl+ (aq) ions because [1996]
a)
H+ has lower hydration enthalpy
b)
Cl+ has lower hydration enthalpy
c)
Cl has high electron a ffinity
d)
Cl has high electron egativity

|
Ruchi Chopra answered |
Hydration energy of Cl+ is very less than H+ hence it doesn’t form Cl+ (aq) ion.
The densities of graphite and diamond at 298 K are 2.25 and 3.31g cm–3, respectively. If the standard free energy difference (ΔGº) is equal to 1895 J mol–1, the pressure at which graphite will be transformed into diamond at 298 K is [2003]- a)9.92 × 105 Pa
- b)9.92 × 108 Pa
- c)9.92 × 107 Pa
- d)9.92 × 106 Pa
Correct answer is option 'B'. Can you explain this answer?
The densities of graphite and diamond at 298 K are 2.25 and 3.31g cm–3, respectively. If the standard free energy difference (ΔGº) is equal to 1895 J mol–1, the pressure at which graphite will be transformed into diamond at 298 K is [2003]
a)
9.92 × 105 Pa
b)
9.92 × 108 Pa
c)
9.92 × 107 Pa
d)
9.92 × 106 Pa

|
Snehal Shah answered |
ΔG = – PΔV = Work done
ΔV is the change in molar volume in the conversion of graphite to diamond.
ΔV is the change in molar volume in the conversion of graphite to diamond.
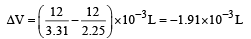
Work done = –(–1.91 × 10–3) × P × 101.3 J
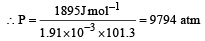
1 atm = 105 × 1.013 Pa
⇒ P = 9.92 x 108 Pa
Given that bond energies of H – H and Cl – Cl are 430 kJ mol– 1 and 240 kJ mol–1 respectively and ΔHf for HCl is – 90 kJ mol– 1, bond enthalpy of HCl is[2007]- a)380 kJ mol–1
- b)425 kJ mol–1
- c)245 kJ mol–1
- d)290 kJ mol–1
Correct answer is option 'B'. Can you explain this answer?
Given that bond energies of H – H and Cl – Cl are 430 kJ mol– 1 and 240 kJ mol–1 respectively and ΔHf for HCl is – 90 kJ mol– 1, bond enthalpy of HCl is[2007]
a)
380 kJ mol–1
b)
425 kJ mol–1
c)
245 kJ mol–1
d)
290 kJ mol–1
|
|
Jananignanamurugan Janan answered |

Considering entropy(S) as a thermodynamic parameter, the criterion for the spontaneity of any process is [2004]- a)ΔSsystem + ΔSsurroundings > 0
- b)ΔSsystem - ΔSsurroundings > 0
- c)ΔSsystem > 0 only
- d)ΔSsurroundings > 0 only
Correct answer is option 'A'. Can you explain this answer?
Considering entropy(S) as a thermodynamic parameter, the criterion for the spontaneity of any process is [2004]
a)
ΔSsystem + ΔSsurroundings > 0
b)
ΔSsystem - ΔSsurroundings > 0
c)
ΔSsystem > 0 only
d)
ΔSsurroundings > 0 only

|
Aashna Mukherjee answered |
For a spon taneous process, ΔStotal is always positive
What is the entropy change (in JK–1 mol–1) when one mole of ice is converted into water at 0º C? (The enthalpy change for the conversion of ice to liquid water is 6.0 kJ mol–1 at 0ºC) [2003]- a)21.98
- b)20.13
- c)2.013
- d)2.198
Correct answer is option 'A'. Can you explain this answer?
What is the entropy change (in JK–1 mol–1) when one mole of ice is converted into water at 0º C? (The enthalpy change for the conversion of ice to liquid water is 6.0 kJ mol–1 at 0ºC) [2003]
a)
21.98
b)
20.13
c)
2.013
d)
2.198

|
Subhankar Datta answered |
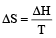
ΔS (per mole )

= 21.98 JK -1mol-1
Standard enthalpy and standard entropy changes for the oxidation of ammonia at 298 K are – 382.64 kJ mol–1 and -145.6 JK–1 mol–1, respectively.Standard Gibb's energy change for the same reaction at 298 K is [2004] - a)-22.1 kJ mol–1
- b)- 339.3 kJ mol–1
- c)- 439.3 kJ mol–1
- d)- 523.2 kJ mol–1
Correct answer is option 'B'. Can you explain this answer?
Standard enthalpy and standard entropy changes for the oxidation of ammonia at 298 K are – 382.64 kJ mol–1 and -145.6 JK–1 mol–1, respectively.Standard Gibb's energy change for the same reaction at 298 K is [2004]
a)
-22.1 kJ mol–1
b)
- 339.3 kJ mol–1
c)
- 439.3 kJ mol–1
d)
- 523.2 kJ mol–1

|
Krish Saha answered |
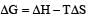
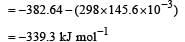
If the enthalpy change for the transition of liquid water to steam is 30 kJ mol–1 at 27ºC, the entropy change for the process would be : [2011]
- a)10 J mol–1 K–1
- b)1.0 J mol–1 K–1
- c)0.1 J mol–1 K–1
- d)100J mol–1 K–1
Correct answer is option 'D'. Can you explain this answer?
If the enthalpy change for the transition of liquid water to steam is 30 kJ mol–1 at 27ºC, the entropy change for the process would be : [2011]
a)
10 J mol–1 K–1
b)
1.0 J mol–1 K–1
c)
0.1 J mol–1 K–1
d)
100J mol–1 K–1
|
|
Surendra Bishnoi answered |
Entropy =enthaply /temperature (kelvin) so enthaply =30kJ=30000J so entropy =30,000/300=100J
Assume each reaction is carried out in an open container. For which reaction will ΔH = ΔE ?- a)C(s) + 2H2O (g) → 2H2 (g) + CO2 (g)
- b)PCl5 (g) → PCl3 (g) + Cl2 (g) [2006]
- c)2CO (g) + O2 (g) → 2CO2 (g)
- d)H2 (g) + Br2 (g) → 2 HBr (g)
Correct answer is option 'D'. Can you explain this answer?
Assume each reaction is carried out in an open container. For which reaction will ΔH = ΔE ?
a)
C(s) + 2H2O (g) → 2H2 (g) + CO2 (g)
b)
PCl5 (g) → PCl3 (g) + Cl2 (g) [2006]
c)
2CO (g) + O2 (g) → 2CO2 (g)
d)
H2 (g) + Br2 (g) → 2 HBr (g)
|
|
Tanvi Kulkarni answered |
The volume decrease?
A. 2H2(g) + O2(g) → 2H2O(g)
B. 2H2O(g) → 2H2(g) + O2(g)
C. 2H2(g) + Cl2(g) → 2HCl(g)
D. 2HCl(g) → 2H2(g) + Cl2(g)
To determine which reaction will result in a decrease in volume, we need to analyze the stoichiometry of the reactions. The coefficients of the reactants and products indicate the relative number of molecules involved in the reaction.
A decrease in volume would occur if the number of moles of gaseous reactants is greater than the number of moles of gaseous products. This means that the total number of gaseous molecules is decreasing, resulting in a decrease in volume.
Let's analyze each reaction:
A. 2H2(g) + O2(g) → 2H2O(g)
In this reaction, 2 moles of hydrogen gas combine with 1 mole of oxygen gas to produce 2 moles of water vapor. The number of moles of gaseous reactants is 2 + 1 = 3, while the number of moles of gaseous products is 2. Therefore, the total number of gaseous molecules is decreasing, and the volume will decrease.
B. 2H2O(g) → 2H2(g) + O2(g)
In this reaction, 2 moles of water vapor decompose to form 2 moles of hydrogen gas and 1 mole of oxygen gas. The number of moles of gaseous reactants is 2, while the number of moles of gaseous products is 2 + 1 = 3. Therefore, the total number of gaseous molecules is increasing, and the volume will not decrease.
C. 2H2(g) + Cl2(g) → 2HCl(g)
In this reaction, 2 moles of hydrogen gas react with 1 mole of chlorine gas to produce 2 moles of hydrogen chloride gas. The number of moles of gaseous reactants is 2 + 1 = 3, while the number of moles of gaseous products is 2. Therefore, the total number of gaseous molecules is decreasing, and the volume will decrease.
D. 2HCl(g) → 2H2(g) + Cl2(g)
In this reaction, 2 moles of hydrogen chloride gas decompose to form 2 moles of hydrogen gas and 1 mole of chlorine gas. The number of moles of gaseous reactants is 2, while the number of moles of gaseous products is 2 + 1 = 3. Therefore, the total number of gaseous molecules is increasing, and the volume will not decrease.
Based on this analysis, the reactions that will result in a decrease in volume are A and C.
A. 2H2(g) + O2(g) → 2H2O(g)
B. 2H2O(g) → 2H2(g) + O2(g)
C. 2H2(g) + Cl2(g) → 2HCl(g)
D. 2HCl(g) → 2H2(g) + Cl2(g)
To determine which reaction will result in a decrease in volume, we need to analyze the stoichiometry of the reactions. The coefficients of the reactants and products indicate the relative number of molecules involved in the reaction.
A decrease in volume would occur if the number of moles of gaseous reactants is greater than the number of moles of gaseous products. This means that the total number of gaseous molecules is decreasing, resulting in a decrease in volume.
Let's analyze each reaction:
A. 2H2(g) + O2(g) → 2H2O(g)
In this reaction, 2 moles of hydrogen gas combine with 1 mole of oxygen gas to produce 2 moles of water vapor. The number of moles of gaseous reactants is 2 + 1 = 3, while the number of moles of gaseous products is 2. Therefore, the total number of gaseous molecules is decreasing, and the volume will decrease.
B. 2H2O(g) → 2H2(g) + O2(g)
In this reaction, 2 moles of water vapor decompose to form 2 moles of hydrogen gas and 1 mole of oxygen gas. The number of moles of gaseous reactants is 2, while the number of moles of gaseous products is 2 + 1 = 3. Therefore, the total number of gaseous molecules is increasing, and the volume will not decrease.
C. 2H2(g) + Cl2(g) → 2HCl(g)
In this reaction, 2 moles of hydrogen gas react with 1 mole of chlorine gas to produce 2 moles of hydrogen chloride gas. The number of moles of gaseous reactants is 2 + 1 = 3, while the number of moles of gaseous products is 2. Therefore, the total number of gaseous molecules is decreasing, and the volume will decrease.
D. 2HCl(g) → 2H2(g) + Cl2(g)
In this reaction, 2 moles of hydrogen chloride gas decompose to form 2 moles of hydrogen gas and 1 mole of chlorine gas. The number of moles of gaseous reactants is 2, while the number of moles of gaseous products is 2 + 1 = 3. Therefore, the total number of gaseous molecules is increasing, and the volume will not decrease.
Based on this analysis, the reactions that will result in a decrease in volume are A and C.
For vapor ization of water at 1 atmospheric pressure, the values of ΔH and ΔS are 40.63 kJmol–1 and 108.8 JK–1 mol–1, respectively. The temperature when Gibbs energy change (ΔG) for this transformation will be zero, is: [2010]- a)293.4 K
- b)273.4 K
- c)393.4 K
- d)373.4 K.
Correct answer is option 'D'. Can you explain this answer?
For vapor ization of water at 1 atmospheric pressure, the values of ΔH and ΔS are 40.63 kJmol–1 and 108.8 JK–1 mol–1, respectively. The temperature when Gibbs energy change (ΔG) for this transformation will be zero, is: [2010]
a)
293.4 K
b)
273.4 K
c)
393.4 K
d)
373.4 K.

|
Ishani Nambiar answered |
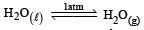
ΔH = 40630J mol –1
ΔS = 108.8JK–1 mol –1
ΔS = 108.8JK–1 mol –1
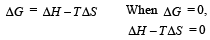
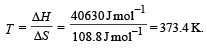
∴ Correct choice : (d)
Standard entropies of X2 , Y2 and XY3 are 60, 40 and 50 JK–1mol–1 respectively. For the reaction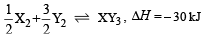
to be at equilibrium, the temperature should be:- a)750 K
- b)1000 K [2010]
- c)1250 K
- d)500 K
Correct answer is option 'A'. Can you explain this answer?
Standard entropies of X2 , Y2 and XY3 are 60, 40 and 50 JK–1mol–1 respectively. For the reaction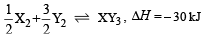
to be at equilibrium, the temperature should be:
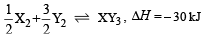
to be at equilibrium, the temperature should be:
a)
750 K
b)
1000 K [2010]
c)
1250 K
d)
500 K

|
Devansh Mehra answered |
ΔS for the reaction 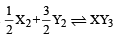
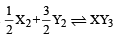
ΔS = 50 – (30 + 60) = – 40 J
For equilibrium ΔG = 0 = ΔH – TΔS
For equilibrium ΔG = 0 = ΔH – TΔS
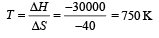
Unit of entropy is [2002]- a)JK–1 mol–1
- b)J mol–1
- c)J–1 K–1 mol–1
- d)JK mol–1
Correct answer is option 'A'. Can you explain this answer?
Unit of entropy is [2002]
a)
JK–1 mol–1
b)
J mol–1
c)
J–1 K–1 mol–1
d)
JK mol–1

|
Pallabi Reddy answered |
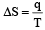
q —→ required heat per mole
T —→ constant absolute temperature
Unit of entropy is JK–1 mol–1
T —→ constant absolute temperature
Unit of entropy is JK–1 mol–1
Identify the correct statement regarding entropy: [1998]- a)At absolute zero of temper ature, entropy of a perfectly crystalline substance is taken to be zero
- b)At absolute zero of temperature, the entropy of a perfectly crystalline substance is +ve
- c)At absolute zero of temperature, the entropy of all crystalline substances is taken to be zero
- d)At 0ºC, the entropy of a perfectly crystalline substance is taken to be zero
Correct answer is option 'A'. Can you explain this answer?
Identify the correct statement regarding entropy: [1998]
a)
At absolute zero of temper ature, entropy of a perfectly crystalline substance is taken to be zero
b)
At absolute zero of temperature, the entropy of a perfectly crystalline substance is +ve
c)
At absolute zero of temperature, the entropy of all crystalline substances is taken to be zero
d)
At 0ºC, the entropy of a perfectly crystalline substance is taken to be zero

|
Ishaan Menon answered |
We know from the third law of thermodynamics, the entropy of a perfectly crystalline substance at absolute zero temperature is taken to be zero.
Standard enthalpy of vapourisation Δvap H° for water at 100°C is 40.66 kJ mol–1. The internal energy of vaporisation of water at 100°C (in kJ mol–1) is : [2012](Assume water vapour to behave like an ideal gas).- a)+ 40.66
- b)– 43.76
- c)+ 43.76
- d)+ 37.56
Correct answer is option 'D'. Can you explain this answer?
Standard enthalpy of vapourisation Δvap H° for water at 100°C is 40.66 kJ mol–1. The internal energy of vaporisation of water at 100°C (in kJ mol–1) is : [2012]
(Assume water vapour to behave like an ideal gas).
a)
+ 40.66
b)
– 43.76
c)
+ 43.76
d)
+ 37.56
|
|
Anoushka Yadav answered |
∆vapH° = 40.66 kJ mol-1
H2O(l) ⇋ H2O(g)
∆ng = 1-0 = 1
∆H = ∆U + ∆ngRT
∆U = ∆H-∆ngRT
= 40,660 - 8.314×373
= 37558.878 J mol-1 or 37.56kJ mol-1
H2O(l) ⇋ H2O(g)
∆ng = 1-0 = 1
∆H = ∆U + ∆ngRT
∆U = ∆H-∆ngRT
= 40,660 - 8.314×373
= 37558.878 J mol-1 or 37.56kJ mol-1
From the following bond energies: [2009]
H – H bond energy: 431.37 kJ mol–1
C = C bond energy: 606.10 kJ mol–1
C – C bond energy: 336.49 kJ mol–1
C – H bond energy: 410.50 kJ mol–1
Enthalpy for the reaction,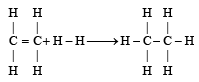 will be:
will be:- a)– 243.6 kJ mol–1
- b)–120.0 kJ mol–1
- c)553.0 kJ mol–1
- d)1523.6 kJ mol–1
Correct answer is option 'B'. Can you explain this answer?
From the following bond energies: [2009]
H – H bond energy: 431.37 kJ mol–1
C = C bond energy: 606.10 kJ mol–1
C – C bond energy: 336.49 kJ mol–1
C – H bond energy: 410.50 kJ mol–1
Enthalpy for the reaction,
H – H bond energy: 431.37 kJ mol–1
C = C bond energy: 606.10 kJ mol–1
C – C bond energy: 336.49 kJ mol–1
C – H bond energy: 410.50 kJ mol–1
Enthalpy for the reaction,
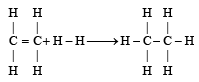
will be:
a)
– 243.6 kJ mol–1
b)
–120.0 kJ mol–1
c)
553.0 kJ mol–1
d)
1523.6 kJ mol–1

|
Swara Desai answered |
Enthalpy of reaction = B.E(Reactant)– B.E(Product)
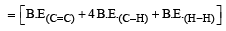
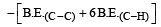
= [606.1 + (4 × 410.5) + 431.37)] – [336.49
+ (6 × 410.5)]
= –120.0 kJ mol–1
+ (6 × 410.5)]
= –120.0 kJ mol–1
For which one of the following equations is ΔHºreact equal to ΔHfº for the product? [2003]- a)
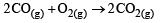
- b)
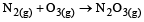
- c)
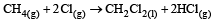
- d)
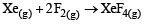
Correct answer is option 'D'. Can you explain this answer?
For which one of the following equations is ΔHºreact equal to ΔHfº for the product? [2003]
a)
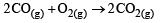
b)
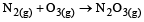
c)
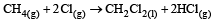
d)
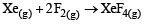
|
|
Niharika Nair answered |
The heat of formation is defined as the heat generated or observed when the compound is formed from its component elements in their standard state.
By the definition (C) and (B) are incorrect as the starting material are compound.
(A) is incorrect as ozone is not the standard state for carbon.
Now (D) is correct as it satisfies the definition of heat of formation.
When 1 mol of a gas is heated at constant volume, temperature is raised from 298 to 308 K. If heat supplied to the gas is 500 J, then which statement is correct ? [2001]- a)q = w = 500 J, ΔU = 0
- b)q = ΔU = 500 J, w = 0
- c)q = –w = 500 J, U = 0
- d)ΔU = 0, q = w = –500 J
Correct answer is option 'B'. Can you explain this answer?
When 1 mol of a gas is heated at constant volume, temperature is raised from 298 to 308 K. If heat supplied to the gas is 500 J, then which statement is correct ? [2001]
a)
q = w = 500 J, ΔU = 0
b)
q = ΔU = 500 J, w = 0
c)
q = –w = 500 J, U = 0
d)
ΔU = 0, q = w = –500 J

|
Pooja Saha answered |
As volume is constant hence work done in this proces is zero hence heat supplied is equal to change in internal energy.
The Gibbs’ energy for the decomposition of Al2O3 at 500°C is as follows :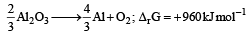
The potential difference needed for the electrolytic reduction of aluminium oxide (Al2O3) at 500°C is at least : [2012 M]- a)4.5 V
- b)3.0 V
- c)2.5 V
- d)5.0 V
Correct answer is option 'C'. Can you explain this answer?
The Gibbs’ energy for the decomposition of Al2O3 at 500°C is as follows :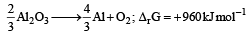
The potential difference needed for the electrolytic reduction of aluminium oxide (Al2O3) at 500°C is at least : [2012 M]
The potential difference needed for the electrolytic reduction of aluminium oxide (Al2O3) at 500°C is at least : [2012 M]
a)
4.5 V
b)
3.0 V
c)
2.5 V
d)
5.0 V

|
Pooja Saha answered |
ΔG = -nFE°
for the reaction n 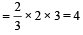
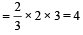
960 × 103 = – 4 × 96500 × E°
E° = – 2.5 volt
So, it needed 2.5 volt for reduction
E° = – 2.5 volt
So, it needed 2.5 volt for reduction
Standard Gibb’s free energy change for isomerization reaction [1995] cis-2 pentene  trans-2-pentene is – 3.67 kJ/mol at 400 K. If more trans-2 pentene is added to the reaction vessel, then
trans-2-pentene is – 3.67 kJ/mol at 400 K. If more trans-2 pentene is added to the reaction vessel, then- a)more cis-2 pentene is formed
- b)equilibrium remains unaffected
- c)additional trans-2 pentene is formed
- d)equilibrium is shifted in forward direction
Correct answer is option 'A'. Can you explain this answer?
Standard Gibb’s free energy change for isomerization reaction [1995] cis-2 pentene  trans-2-pentene is – 3.67 kJ/mol at 400 K. If more trans-2 pentene is added to the reaction vessel, then
trans-2-pentene is – 3.67 kJ/mol at 400 K. If more trans-2 pentene is added to the reaction vessel, then
 trans-2-pentene is – 3.67 kJ/mol at 400 K. If more trans-2 pentene is added to the reaction vessel, then
trans-2-pentene is – 3.67 kJ/mol at 400 K. If more trans-2 pentene is added to the reaction vessel, thena)
more cis-2 pentene is formed
b)
equilibrium remains unaffected
c)
additional trans-2 pentene is formed
d)
equilibrium is shifted in forward direction

|
Prasenjit Pillai answered |
If more tran s-2-pentene is added, then its concentration in right hand side will increase. But in order to maintain the K constant, concentration of cis-2-pentene will also increase. Therefore more cis-2-pentene will be formed.
Chapter doubts & questions for Thermodynamics - NEET Past Year Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermodynamics - NEET Past Year Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily