All Exams >
NEET >
NEET Past Year Papers >
All Questions
All questions of Atoms for NEET Exam
Which of the following transitions in a hydrogenatom emits the photon of highest frequency? [2000]- a)n = 2 to n = 1
- b)n = 2 to n = 6
- c)n = 6 to n = 2
- d)n = 1 to n = 2
Correct answer is option 'A'. Can you explain this answer?
Which of the following transitions in a hydrogenatom emits the photon of highest frequency? [2000]
a)
n = 2 to n = 1
b)
n = 2 to n = 6
c)
n = 6 to n = 2
d)
n = 1 to n = 2

|
Naveen Menon answered |
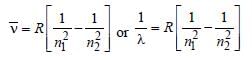
Frequency, 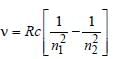
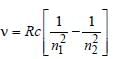
Note : See the greatest energy difference and
also see that the transition is from higher to
lower energy level. Hence, it is highest in
case of n = 2 to n = 1.
also see that the transition is from higher to
lower energy level. Hence, it is highest in
case of n = 2 to n = 1.
J.J. Thomson’s cathode-ray tube experimentdemonstrated that [2003]- a)the e/m ratio of the cathode-ray particleschanges when a different gas is placed inthe discharge tube
- b)cathode rays are streams of negatively
charged ions - c)all the mass of an atom is essentially in the
nucleus - d)the e/m of electrons is much greater thanthe e/m of protons
Correct answer is option 'B'. Can you explain this answer?
J.J. Thomson’s cathode-ray tube experimentdemonstrated that [2003]
a)
the e/m ratio of the cathode-ray particleschanges when a different gas is placed inthe discharge tube
b)
cathode rays are streams of negatively
charged ions
charged ions
c)
all the mass of an atom is essentially in the
nucleus
nucleus
d)
the e/m of electrons is much greater thanthe e/m of protons

|
Prasenjit Pillai answered |
Cathode rays are streams of negatively
charged ions
charged ions
The half life of radium is about 1600 years. Of 100 g of radium existing now, 25 g will remainunchanged after [2004]- a)3200 years
- b)4800 years
- c)6400 years
- d)2400 years
Correct answer is option 'A'. Can you explain this answer?
The half life of radium is about 1600 years. Of 100 g of radium existing now, 25 g will remainunchanged after [2004]
a)
3200 years
b)
4800 years
c)
6400 years
d)
2400 years

|
Maheshwar Saini answered |
100 g will become 25 g in two half lives, so, it
is 3200 years
is 3200 years
What is the radius of iodine atom (At. no. 53,mass no. 126) [1988]- a)2.5 × 10–11 m
- b)2.5 × 10–9 m
- c)7 × 10–9 m
- d)7 × 10–6 m
Correct answer is option 'A'. Can you explain this answer?
What is the radius of iodine atom (At. no. 53,mass no. 126) [1988]
a)
2.5 × 10–11 m
b)
2.5 × 10–9 m
c)
7 × 10–9 m
d)
7 × 10–6 m

|
Sneha Basak answered |
53 electrons in iodine atom are distributed as
2, 8, 18, 18, 7
∴ n = 5
2, 8, 18, 18, 7
∴ n = 5
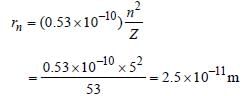
In Rutherford scattering experiment, what willbe the correct angle for α-scattering for an impactparameter, b = 0 ? [1994]- a)90°
- b)270°
- c)0°
- d)180°
Correct answer is option 'D'. Can you explain this answer?
In Rutherford scattering experiment, what willbe the correct angle for α-scattering for an impactparameter, b = 0 ? [1994]
a)
90°
b)
270°
c)
0°
d)
180°

|
Abhijeet Goyal answered |
Impact parameter for Rutherford scattering
experiment,
experiment,
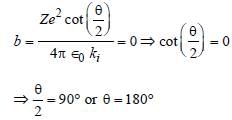
The spectrum obtained from a sodium vapourlamp is an example of [1995]- a)band spectrum
- b)continuous spectrum
- c)emission spectrum
- d)absorption spectrum
Correct answer is option 'C'. Can you explain this answer?
The spectrum obtained from a sodium vapourlamp is an example of [1995]
a)
band spectrum
b)
continuous spectrum
c)
emission spectrum
d)
absorption spectrum

|
Aniket Chawla answered |
A spectrum is observed, when light coming
directly from a source is examined with a
spectroscope. Therefore spectrum obtained
from a sodium vapour lamp is emission
spectrum.
directly from a source is examined with a
spectroscope. Therefore spectrum obtained
from a sodium vapour lamp is emission
spectrum.
The transition from the state n = 3 to n = 1 in ahydrogen like atom results in ultravioletradiation. Infrared radiation will be obtained inthe transition from : [2012M]- a)2 → 1
- b)3 → 2
- c)4 → 2
- d)4 → 3
Correct answer is option 'D'. Can you explain this answer?
The transition from the state n = 3 to n = 1 in ahydrogen like atom results in ultravioletradiation. Infrared radiation will be obtained inthe transition from : [2012M]
a)
2 → 1
b)
3 → 2
c)
4 → 2
d)
4 → 3

|
Deepak Joshi answered |
The frequency of the transition 
when n = 1, 2, 3.
Which source is associated with a line emissionspectrum ? [1993]- a)Electric fire
- b)Neon street sign
- c)Red traffic light
- d)sun
Correct answer is option 'B'. Can you explain this answer?
Which source is associated with a line emissionspectrum ? [1993]
a)
Electric fire
b)
Neon street sign
c)
Red traffic light
d)
sun

|
Ayush Chavan answered |
Neon street sign is a source of line emission
spectrum.
spectrum.
The total energy of electron in the ground stateof hydrogen atom is – 13.6 eV. The kinetic energyof an electron in the first excited state is [2007]- a)6.8 eV
- b)13.6 eV
- c)1.7 eV
- d)3.4 eV.
Correct answer is option 'D'. Can you explain this answer?
The total energy of electron in the ground stateof hydrogen atom is – 13.6 eV. The kinetic energyof an electron in the first excited state is [2007]
a)
6.8 eV
b)
13.6 eV
c)
1.7 eV
d)
3.4 eV.

|
Srishti Sen answered |
Energy in the first excited state
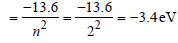
But K.E. = –(Total energy) = +3.4 eV.
When a hydrogen atom is raised from the groundstate to an excited state, [1995]- a)P.E decreases and K.E. increases
- b)P.E. increases and K.E decreases
- c)both K.E. and P.E. decrease
- d)absorption spectrum
Correct answer is option 'B'. Can you explain this answer?
When a hydrogen atom is raised from the groundstate to an excited state, [1995]
a)
P.E decreases and K.E. increases
b)
P.E. increases and K.E decreases
c)
both K.E. and P.E. decrease
d)
absorption spectrum

|
Arnav Iyer answered |
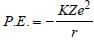 and
and 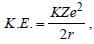 where,
where,r is the radius of orbit which increases as we
move from ground to an excited state.
Therefore, when a hydrogen atom is raised
from the ground state, it increases the value
of r. As a result of this, P.E. increases
(decreases in negative) and K.E. decreases.
If the threshold wavelength for a certain metal is 2000 Å, then the work-function of the metal is [1995]- a)6.2 J
- b)6.2 eV [
- c)6.2 MeV
- d)6.2 keV
Correct answer is option 'B'. Can you explain this answer?
If the threshold wavelength for a certain metal is 2000 Å, then the work-function of the metal is [1995]
a)
6.2 J
b)
6.2 eV [
c)
6.2 MeV
d)
6.2 keV

|
Raghav Khanna answered |
Threshold wavelength (λ) = 2000 Å = 2000 ×
10–10 m. Work function
10–10 m. Work function
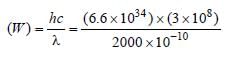

When an electron jumps from the fourth orbit tothe second orbit, one gets the [2000]- a)second line of Lyman series
- b)second line of Paschen series
- c)second line of Balmer series
- d)first line of Pfund series
Correct answer is option 'C'. Can you explain this answer?
When an electron jumps from the fourth orbit tothe second orbit, one gets the [2000]
a)
second line of Lyman series
b)
second line of Paschen series
c)
second line of Balmer series
d)
first line of Pfund series

|
Lekshmi Banerjee answered |
When the electron drops from any orbit to
second orbit, then wavelength of line
obtained belongs to Balmer series.
second orbit, then wavelength of line
obtained belongs to Balmer series.
The total energy of an electron in the firstexcited state of hydrogen atom is about –3.4eV. Its kinetic energy in this state is [2005]- a)3.4 eV
- b)6.8 eV
- c)–3.4 eV
- d)–6.8 eV
Correct answer is option 'A'. Can you explain this answer?
The total energy of an electron in the firstexcited state of hydrogen atom is about –3.4eV. Its kinetic energy in this state is [2005]
a)
3.4 eV
b)
6.8 eV
c)
–3.4 eV
d)
–6.8 eV

|
Aniket Chawla answered |
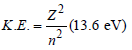
Mechanical energy =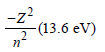
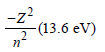
∴ K.E. in 2nd orbital for hydrogen
= – Mechanical energy
= – Mechanical energy
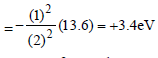
Ionization potential of hydrogen atom is 13.6eV.Hydrogen atoms in the ground state are excitedby monochromatic radiation of photon energy12.1 eV. According to Bohr’s theory, the spectrallines emitted by hydrogen will be [2006]- a)three
- b)Four
- c)One
- d)Two
Correct answer is option 'A'. Can you explain this answer?
Ionization potential of hydrogen atom is 13.6eV.Hydrogen atoms in the ground state are excitedby monochromatic radiation of photon energy12.1 eV. According to Bohr’s theory, the spectrallines emitted by hydrogen will be [2006]
a)
three
b)
Four
c)
One
d)
Two

|
Abhishek Choudhary answered |
Energy of ground state 13.6 eV
Energy of first excited state
Energy of first excited state
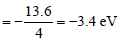
Energy of second excited state
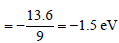
Difference between ground state and 2nd
excited state = 13.6 – 1.5 = 12.1 eV
So, electron can be excited upto 3rd orbit
No. of possible transition
1 → 2, 1 → 3, 2 → 3
So, three lines are possible
excited state = 13.6 – 1.5 = 12.1 eV
So, electron can be excited upto 3rd orbit
No. of possible transition
1 → 2, 1 → 3, 2 → 3
So, three lines are possible
The ionization energy of the electron in thehydrogen atom in its ground state is 13.6 eV.The atoms are excited to higher energy levels toemit radiations of 6 wavelengths. Maximumwavelength of emitted radiation corresponds tothe transition between [2009]- a)n = 3 to n = 1 states
- b)n = 2 to n = 1 states
- c)n = 4 to n = 3 states
- d)n = 3 to n = 2 states
Correct answer is option 'C'. Can you explain this answer?
The ionization energy of the electron in thehydrogen atom in its ground state is 13.6 eV.The atoms are excited to higher energy levels toemit radiations of 6 wavelengths. Maximumwavelength of emitted radiation corresponds tothe transition between [2009]
a)
n = 3 to n = 1 states
b)
n = 2 to n = 1 states
c)
n = 4 to n = 3 states
d)
n = 3 to n = 2 states
|
|
Srishti Chavan answered |
Explanation:
When hydrogen atoms are excited to higher energy levels, they release energy in the form of photons. The energy of a photon is given by the equation:
E = hc/λ
where E is the energy of the photon, h is Planck's constant, c is the speed of light, and λ is the wavelength of the photon.
The maximum wavelength of the emitted radiation corresponds to the transition that releases the least amount of energy. This occurs when the electron transitions from a higher energy level to the ground state.
To determine which transition corresponds to the maximum wavelength of the emitted radiation, we can use the equation:
ΔE = -Rh/n^2f + Rh/n^2i
where ΔE is the energy released during the transition, Rh is the Rydberg constant (2.18 x 10^-18 J), nif is the initial energy level, and nf is the final energy level.
We want to find the transition that releases the least amount of energy, which corresponds to the maximum wavelength of the emitted radiation. This occurs when nf is as large as possible and nif is as small as possible.
Using this approach, we find that the transition between n = 4 and n = 3 states releases the least amount of energy, and therefore corresponds to the maximum wavelength of the emitted radiation. The correct answer is option C.
When hydrogen atoms are excited to higher energy levels, they release energy in the form of photons. The energy of a photon is given by the equation:
E = hc/λ
where E is the energy of the photon, h is Planck's constant, c is the speed of light, and λ is the wavelength of the photon.
The maximum wavelength of the emitted radiation corresponds to the transition that releases the least amount of energy. This occurs when the electron transitions from a higher energy level to the ground state.
To determine which transition corresponds to the maximum wavelength of the emitted radiation, we can use the equation:
ΔE = -Rh/n^2f + Rh/n^2i
where ΔE is the energy released during the transition, Rh is the Rydberg constant (2.18 x 10^-18 J), nif is the initial energy level, and nf is the final energy level.
We want to find the transition that releases the least amount of energy, which corresponds to the maximum wavelength of the emitted radiation. This occurs when nf is as large as possible and nif is as small as possible.
Using this approach, we find that the transition between n = 4 and n = 3 states releases the least amount of energy, and therefore corresponds to the maximum wavelength of the emitted radiation. The correct answer is option C.
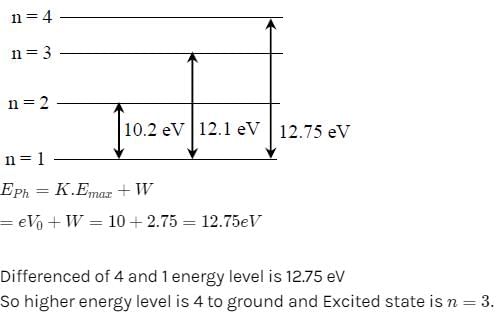
An electron in the hydrogen atom jumps from excited state n to the ground state. The wavelength so emitted illuminates a
photosensitive material having work function 2.75 eV. If the stopping potential of thephotoelectron is 10 V, the value of n is [2011 M]- a)4
- b)3
- c)5
- d)2
Correct answer is option 'B'. Can you explain this answer?
An electron in the hydrogen atom jumps from excited state n to the ground state. The wavelength so emitted illuminates a
photosensitive material having work function 2.75 eV. If the stopping potential of thephotoelectron is 10 V, the value of n is [2011 M]
photosensitive material having work function 2.75 eV. If the stopping potential of thephotoelectron is 10 V, the value of n is [2011 M]
a)
4
b)
3
c)
5
d)
2
|
|
Gowri Menon answered |
In a Rutherford scattering experiment when a projectile of charge Z1 and mass M1 approaches a target nucleus of charge Z2 and mass M2, the distance of closest approach is r0. The energy of the projectile is [2009]- a)directly proportional to Z1 Z2
- b)inversely proportional to Z1
- c)directly proportional to mass M1
- d)directly proportional to M1 × M2
Correct answer is option 'A'. Can you explain this answer?
In a Rutherford scattering experiment when a projectile of charge Z1 and mass M1 approaches a target nucleus of charge Z2 and mass M2, the distance of closest approach is r0. The energy of the projectile is [2009]
a)
directly proportional to Z1 Z2
b)
inversely proportional to Z1
c)
directly proportional to mass M1
d)
directly proportional to M1 × M2

|
Sneha Basak answered |
The kinetic energy of the projectile is given by
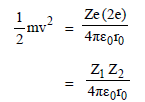
Thus energy of the projectile is directly
proportional to Z1, Z2
proportional to Z1, Z2
The ionisation energy of hydrogen atom is 13.6eV, the ionisation energy of helium atom wouldbe [1988]- a)13.6 eV
- b)27.2 eV
- c)6.8 eV
- d)54.4 eV
Correct answer is option 'D'. Can you explain this answer?
The ionisation energy of hydrogen atom is 13.6eV, the ionisation energy of helium atom wouldbe [1988]
a)
13.6 eV
b)
27.2 eV
c)
6.8 eV
d)
54.4 eV

|
Rajesh Datta answered |
E ∝ Z2 and Z for helium = 2
∴ (E)He = 4x13.6 = 54.4 eV
∴ (E)He = 4x13.6 = 54.4 eV
When hydrogen atom is in its first excited level,its radius is [1997]- a)four times its ground state radius
- b)twice
- c)same
- d)half
Correct answer is option 'A'. Can you explain this answer?
When hydrogen atom is in its first excited level,its radius is [1997]
a)
four times its ground state radius
b)
twice
c)
same
d)
half

|
Shruti Chauhan answered |

Radius in ground state = 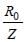
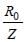
Radius in first excited state = 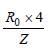
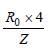
(∵ n =2)
Hence, radius of first excited state is four
times the radius in ground state.
Hence, radius of first excited state is four
times the radius in ground state.
Electron in hydrogen atom first jumps from thirdexcited state to second excited state and thenfrom second excited to the first excited state.The ratio of the wavelength λ1 : λ2 emitted inthe two cases is [2012]- a)7/5
- b)27/20
- c)27/5
- d)20/7
Correct answer is option 'C'. Can you explain this answer?
Electron in hydrogen atom first jumps from thirdexcited state to second excited state and thenfrom second excited to the first excited state.The ratio of the wavelength λ1 : λ2 emitted inthe two cases is [2012]
a)
7/5
b)
27/20
c)
27/5
d)
20/7

|
Kunal Rane answered |
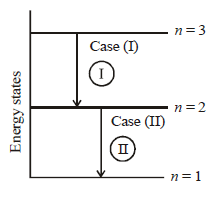
The wave number 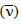 of the radiation = 1/λ
of the radiation = 1/λ
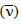 of the radiation = 1/λ
of the radiation = 1/λ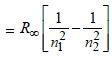
Now for case (I) n1 = 3, n2 = 2
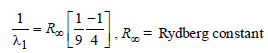
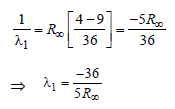
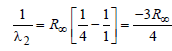
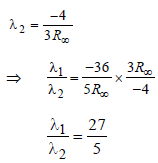
Energy E of a hydrogen atom with principal quantum number n is given by E = – 13.6/n2 eV. The energy of photon ejected when the electronjumps from n = 3 state to n = 2 state of hydrogenis approximately [2004]- a)1.9 eV.
- b)1.5 eV.
- c)0.85 eV
- d)3.4 eV
Correct answer is option 'A'. Can you explain this answer?
Energy E of a hydrogen atom with principal quantum number n is given by E = – 13.6/n2 eV. The energy of photon ejected when the electronjumps from n = 3 state to n = 2 state of hydrogenis approximately [2004]
a)
1.9 eV.
b)
1.5 eV.
c)
0.85 eV
d)
3.4 eV
|
|
Anshu Kaur answered |
-13.6/n^2 eV
An electron in hydrogen atom makes a transition n1 → n2 where n1 and n2 are principal quantum numbers of the two states. Assuming Bohr’s model to be valid the time period of the electron in the initial state is eight times that in the final state. The possible values of n1 and n2 are [NEET Kar. 2013]- a)n1 = 4 and n2 = 2
- b)n1 = 6 and n2 = 2
- c)n1 = 8 and n2 = 1
- d)n1 = 8 and n2 = 2
Correct answer is option 'A'. Can you explain this answer?
An electron in hydrogen atom makes a transition n1 → n2 where n1 and n2 are principal quantum numbers of the two states. Assuming Bohr’s model to be valid the time period of the electron in the initial state is eight times that in the final state. The possible values of n1 and n2 are [NEET Kar. 2013]
a)
n1 = 4 and n2 = 2
b)
n1 = 6 and n2 = 2
c)
n1 = 8 and n2 = 1
d)
n1 = 8 and n2 = 2

|
Maheshwar Saini answered |
T ∝ n3
Tn1 = 8 Tn2 (given)
Hence, n1 = 2n2
Tn1 = 8 Tn2 (given)
Hence, n1 = 2n2
The Bohr model of atoms [2004]- a)predicts the same emission spectra for alltypes of atoms
- b)assumes that the angular momentum ofelectrons is quantised
- c)uses Einstein’s photoelectric equation
- d)predicts continuous emission spectra foratoms
Correct answer is option 'B'. Can you explain this answer?
The Bohr model of atoms [2004]
a)
predicts the same emission spectra for alltypes of atoms
b)
assumes that the angular momentum ofelectrons is quantised
c)
uses Einstein’s photoelectric equation
d)
predicts continuous emission spectra foratoms

|
Arindam Khanna answered |
In Bohr’s model, angular momentum is
quantised i.e =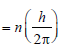
quantised i.e =
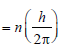
The ratio of longest wavelengths corresponding to Lyman and Blamer series in hydrogen spectrum is [NEET 2013]- a)3/23
- b)7/29
- c)9/31
- d)5/27
Correct answer is option 'D'. Can you explain this answer?
The ratio of longest wavelengths corresponding to Lyman and Blamer series in hydrogen spectrum is [NEET 2013]
a)
3/23
b)
7/29
c)
9/31
d)
5/27

|
Ayush Chavan answered |
For Lyman series (2 → 1)
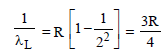
For Balmer series (3 → 2)
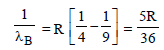
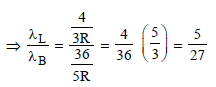
Who indirectly determined the mass of theelectron by measuring the charge of the electron? [2000]- a)Thomson
- b)Rutherford
- c)Einstein
- d)Millikan
Correct answer is option 'D'. Can you explain this answer?
Who indirectly determined the mass of theelectron by measuring the charge of the electron? [2000]
a)
Thomson
b)
Rutherford
c)
Einstein
d)
Millikan
|
|
Snehal Iyer answered |
Correct Answer :- D
Explanation : The mass of the electron is discovered by the Millikan by the oil drop experiment.
E = mg
=> qE = mg
q/m = g/E
Therefore, charge was quantised.
The ground state energy of hydrogen atom is 13.6eV. When its electron is in the first excited state, its excitation energy is [2008]- a)3.4 eV
- b)6.8 eV
- c)10.2 eV
- d)0
Correct answer is option 'C'. Can you explain this answer?
The ground state energy of hydrogen atom is 13.6eV. When its electron is in the first excited state, its excitation energy is [2008]
a)
3.4 eV
b)
6.8 eV
c)
10.2 eV
d)
0

|
Raghav Khanna answered |
When the electron is in first excited state
(n = 2), the excitation energy is given by
(n = 2), the excitation energy is given by
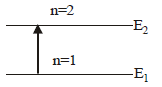
ΔE = E2 – E1
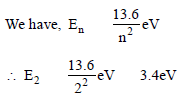
Given E1 = –13.6eV
∴Δ E = ( – 3.4) – ( – 13.6) = 10.2 eV.
∴Δ E = ( – 3.4) – ( – 13.6) = 10.2 eV.
The radius of hydrogen atom in its ground stateis 5.3 × 10–11 m. After collision with an electron itis found to have a radius of 21.2 × 10–11 m. Whatis the principal quantum number n of the finalstate of the atom [1994]- a)n = 4
- b)n = 2
- c)n = 16
- d)n = 3
Correct answer is option 'B'. Can you explain this answer?
The radius of hydrogen atom in its ground stateis 5.3 × 10–11 m. After collision with an electron itis found to have a radius of 21.2 × 10–11 m. Whatis the principal quantum number n of the finalstate of the atom [1994]
a)
n = 4
b)
n = 2
c)
n = 16
d)
n = 3

|
Pooja Choudhary answered |
r ∝ n2
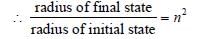
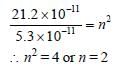
The wavelength of the first line of Lyman seriesfor hydrogen atom is equal to that of the secondline of Balmer series for a hydrogen like ion. Theatomic number Z of hydrogen like ion is [2011]- a)3
- b)4
- c)1
- d)2
Correct answer is option 'D'. Can you explain this answer?
The wavelength of the first line of Lyman seriesfor hydrogen atom is equal to that of the secondline of Balmer series for a hydrogen like ion. Theatomic number Z of hydrogen like ion is [2011]
a)
3
b)
4
c)
1
d)
2

|
Arindam Khanna answered |
For first line of Lyman series of hydrogen
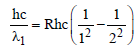
For second line of Balmer series of hydrogen
like ion
like ion
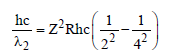
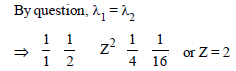
Out of the following which one is not a possibleenergy for a photon to be emitted by hydrogenatom according to Bohr’s atomic model? [2011M]- a)1.9 eV
- b)11.1 eV
- c)13.6 eV
- d)0.65 eV
Correct answer is option 'B'. Can you explain this answer?
Out of the following which one is not a possibleenergy for a photon to be emitted by hydrogenatom according to Bohr’s atomic model? [2011M]
a)
1.9 eV
b)
11.1 eV
c)
13.6 eV
d)
0.65 eV

|
Bhargavi Choudhury answered |
Obviously, difference of 11.1eV is not
possible.
possible.
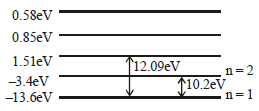
The energy of hydrogen atom in nth orbit is En, then the energy in nth orbit of single ionised helium atom will be [2001]- a)4En
- b)En/4
- c)2En
- d)En/2
Correct answer is option 'A'. Can you explain this answer?
The energy of hydrogen atom in nth orbit is En, then the energy in nth orbit of single ionised helium atom will be [2001]
a)
4En
b)
En/4
c)
2En
d)
En/2

|
Pankaj Kulkarni answered |
We have 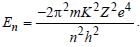 For helium
For helium
Z = 2. Hence requisite answer is 4En.
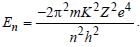 For helium
For heliumZ = 2. Hence requisite answer is 4En.
The energy of a hydrogen atom in the groundstate is – 13.6 eV. The energy of a He+ ion in thefirst excited state will be [2010]- a)–13.6 eV
- b)– 27.2 eV
- c)– 54.4 eV
- d)– 6.8 eV
Correct answer is option 'A'. Can you explain this answer?
The energy of a hydrogen atom in the groundstate is – 13.6 eV. The energy of a He+ ion in thefirst excited state will be [2010]
a)
–13.6 eV
b)
– 27.2 eV
c)
– 54.4 eV
d)
– 6.8 eV

|
Moumita Khanna answered |
Energy of a H-like atom in it's nth state is
given by
given by
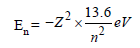
For, first excited state of He+, n = 2, Z = 2
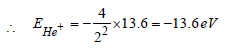
In the Bohr model of a hydrogen atom, the centripetal force is furnished by the coulomb attraction between the proton and the electron. If a0 is the radius of the ground state orbit, m is the mass, e is the charge on the electron and ε0 is the vacuum ermittivity, the speed of the electron is [1998]- a)0
- b)
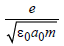
- c)
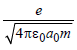
- d)

Correct answer is option 'C'. Can you explain this answer?
In the Bohr model of a hydrogen atom, the centripetal force is furnished by the coulomb attraction between the proton and the electron. If a0 is the radius of the ground state orbit, m is the mass, e is the charge on the electron and ε0 is the vacuum ermittivity, the speed of the electron is [1998]
a)
0
b)
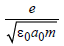
c)
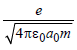
d)


|
Sneha Basak answered |
Centripetal force = Coulombian force
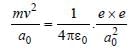
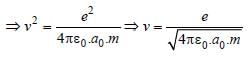
An electron of a stationary hydrogen atom passes from the fifth energy level to the ground level. The velocity that the atom acquired as a result of photon emission will be : [2012] (m is the mass of the electron, R, Rydberg constant and h Planck’s constant)- a)
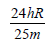
- b)
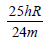
- c)
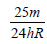
- d)
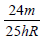
Correct answer is option 'A'. Can you explain this answer?
An electron of a stationary hydrogen atom passes from the fifth energy level to the ground level. The velocity that the atom acquired as a result of photon emission will be : [2012]
(m is the mass of the electron, R, Rydberg constant and h Planck’s constant)
a)
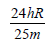
b)
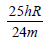
c)
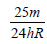
d)
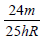

|
Pooja Choudhary answered |
For emission, the wave number of the
radiation is given as
radiation is given as
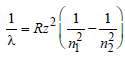
R= Rydberg constant, Z = atomic number
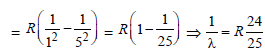
linear momentum
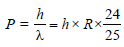 (de-Broglie hypothesis)
(de-Broglie hypothesis)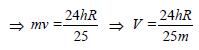
In terms of Bohr radius a0, the radius of the second Bohr orbit of a hydrogen atom is given by [1992]- a)4 a0
- b)8 a0
- c)√2 a0
- d)2 a0
Correct answer is option 'A'. Can you explain this answer?
In terms of Bohr radius a0, the radius of the second Bohr orbit of a hydrogen atom is given by [1992]
a)
4 a0
b)
8 a0
c)
√2 a0
d)
2 a0

|
Dipika Das answered |
As r ∝ n2 , therefore, radius of 2nd
Bohr’s orbit = 4 a0.
Bohr’s orbit = 4 a0.
An alpha nucleus of energy 1/2mv2 bombards a heavy nuclear target of charge Ze. Then the distance of closest approach for the alpha nucleus will be proportional to [2010]- a)1/Ze
- b)v2
- c)1/m
- d)1/v4
Correct answer is option 'C'. Can you explain this answer?
An alpha nucleus of energy 1/2mv2 bombards a heavy nuclear target of charge Ze. Then the distance of closest approach for the alpha nucleus will be proportional to [2010]
a)
1/Ze
b)
v2
c)
1/m
d)
1/v4

|
Shanaya Rane answered |
Kinetic energy of alpha nucleus is equall to
electrostatic potential energy of the system
of the alpha particle and the heavy nucleus.
That is,
electrostatic potential energy of the system
of the alpha particle and the heavy nucleus.
That is,
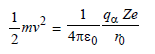
where r0 is the distance of closest approach
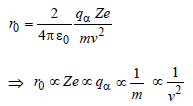
Hence, correct option is (c).
Chapter doubts & questions for Atoms - NEET Past Year Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Atoms - NEET Past Year Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup


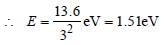

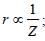 Z(=3) is maximum for Li2+.
Z(=3) is maximum for Li2+.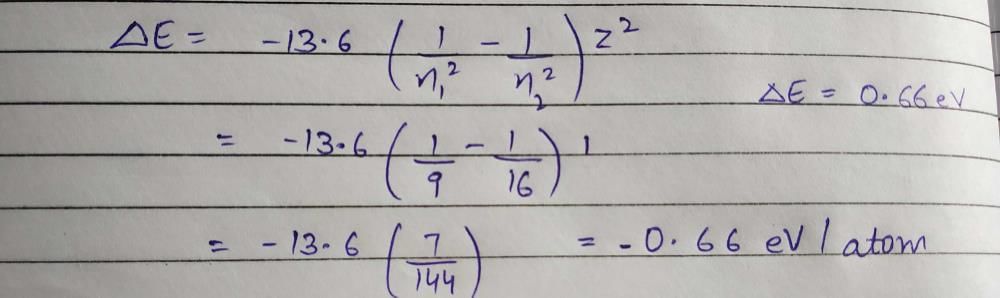
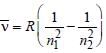 , where n1 = 2, n2 = 4
, where n1 = 2, n2 = 4