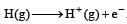All Exams >
NEET >
Inorganic Chemistry for NEET >
All Questions
All questions of Hydrogen for NEET Exam
On an industrial scale, H2O2 is prepared by oxidation of:- a)2-methylanthraquinol
- b)2-ethylanthraquinol
- c)2-ethylanthraquinine
- d)2-ethylanthraquinone
Correct answer is option 'B'. Can you explain this answer?
On an industrial scale, H2O2 is prepared by oxidation of:
a)
2-methylanthraquinol
b)
2-ethylanthraquinol
c)
2-ethylanthraquinine
d)
2-ethylanthraquinone
|
|
Pooja Shah answered |
H2O2 is prepared industrially by oxidation of 2-ethylanthraquinol as it is very cheap.
The O – O – H bond angle in H2O2 is [1994]- a)106°
- b)109°28'
- c)120°
- d)97°
Correct answer is option 'D'. Can you explain this answer?
The O – O – H bond angle in H2O2 is [1994]
a)
106°
b)
109°28'
c)
120°
d)
97°

|
Muskaan Basak answered |
O – O – H bond angle in H2O2 is 97°
Out of NH3,H2O and HF, which has the highest magnitude of Hydrogen bonding:- a)NH3
- b)H2O
- c)HF
- d)All will have the same magnitude
Correct answer is option 'C'. Can you explain this answer?
Out of NH3,H2O and HF, which has the highest magnitude of Hydrogen bonding:
a)
NH3
b)
H2O
c)
HF
d)
All will have the same magnitude
|
|
Nandini Patel answered |
A small and highly electronegativity elements form a stronger hydrogen bond. The order of size of N , O and F is F < O < N .and so the order of strength of hydrogen bond is F > O > N.
Hence, electronegativity of F is the highest, therefore magnitude of positive charge on hydrogen and negative charge on F is the highest in HF and Hence, electrostatic attraction of H bonding is the strongest in HF.
Which of the following is an incorrect statement?- a)Temporary hardness of water is due to chlorides and sulphates of Ca and Mg.
- b)Permanent hardness of water is due to chlorides and sulphates of Ca and Mg.
- c)Temporary hardness of water is due to bicarbonates of Ca and Mg.
- d)Temporary hardness of water can be removed by boiling.
Correct answer is option 'A'. Can you explain this answer?
Which of the following is an incorrect statement?
a)
Temporary hardness of water is due to chlorides and sulphates of Ca and Mg.
b)
Permanent hardness of water is due to chlorides and sulphates of Ca and Mg.
c)
Temporary hardness of water is due to bicarbonates of Ca and Mg.
d)
Temporary hardness of water can be removed by boiling.
|
|
Suresh Iyer answered |
- Temporary hardness is caused by the presence of dissolved bicarbonates of calcium, magnesium, and other heavy metals and the bicarbonates of iron.
- The salts responsible for temporary hardness are Ca(HCO3)2 , Mg(HCO3) Permanent hardness is due to presence of dissolved chlorides and sulphates of calcium, magnesium, iron and other heavy metals.
- Temporary hardness is removed by boiling known as clark’s method. Permanent hardness cannot be removed by boiling so in that case we have to use ion exchange method and calgon’s method.
Which of the following is the product of the given reaction?
P4O10 (s) + 6H2O(I) →- a)H3PO3
- b)H3PO7
- c)H3PO4
- d)H3PO2
Correct answer is option 'C'. Can you explain this answer?
Which of the following is the product of the given reaction?
P4O10 (s) + 6H2O(I) →
P4O10 (s) + 6H2O(I) →
a)
H3PO3
b)
H3PO7
c)
H3PO4
d)
H3PO2
|
|
Riya Banerjee answered |
P4O10 (s) + H2O (l) → H3PO4
Phosphoric acid is a weak acid with the chemical formula H₃PO₄. Orthophosphoric acid refers to phosphoric acid, which is the IUPAC name for this compound. The prefix ortho- is used to distinguish the acid from related phosphoric acids, called polyphosphoric acids.
Phosphoric acid is a weak acid with the chemical formula H₃PO₄. Orthophosphoric acid refers to phosphoric acid, which is the IUPAC name for this compound. The prefix ortho- is used to distinguish the acid from related phosphoric acids, called polyphosphoric acids.
H2O2 can act as:- a)Oxidizing agent
- b)Reducing agent
- c)Both oxidizing and reducing agent
- d)Neither oxidizing nor reducing agent
Correct answer is option 'C'. Can you explain this answer?
H2O2 can act as:
a)
Oxidizing agent
b)
Reducing agent
c)
Both oxidizing and reducing agent
d)
Neither oxidizing nor reducing agent
|
|
Raghav Bansal answered |
Hydrogen peroxide acts as both a reducing agent and oxidizing agent depending upon the nature of the reacting species. When H2O2 serves as an oxidizing agent, the oxygen of hydrogen peroxide (that is present in -1 oxidation state) is reduced to H2O (-2 oxidation state).
10 volume of H2O2 means:- a)1L of this solution will give 10L of O2 at S.T.P
- b)10L of this solution will give 1L of H2 at S.T.P
- c)10L of this solution will give 1L of O2 at S.T.P
- d)1L of this solution will give 10L of H2 at S.T.P
Correct answer is option 'A'. Can you explain this answer?
10 volume of H2O2 means:
a)
1L of this solution will give 10L of O2 at S.T.P
b)
10L of this solution will give 1L of H2 at S.T.P
c)
10L of this solution will give 1L of O2 at S.T.P
d)
1L of this solution will give 10L of H2 at S.T.P
|
|
Anjali Iyer answered |
Volume strength : This tells about the release of oxygen by 1 mL of hydrogen peroxide solution. Like, 10 volume of H2O2 means, 1 mL of this solution will release 10 mL of oxygen.
The sum of number of neutrons and protons in tritium is:- a)5
- b)4
- c)3
- d)6
Correct answer is option 'C'. Can you explain this answer?
The sum of number of neutrons and protons in tritium is:
a)
5
b)
4
c)
3
d)
6
|
|
Arka Desai answered |
**The Sum of Neutrons and Protons in Tritium**
Tritium is a radioactive isotope of hydrogen and has an atomic number of 1. It is denoted by the symbol T or ^3H. The atomic number represents the number of protons in an atom. To determine the sum of neutrons and protons in tritium, we need to know its atomic mass.
**Atomic Mass of Tritium**
The atomic mass of an atom is the sum of its protons and neutrons. Since tritium has an atomic number of 1, we know it has 1 proton. To find the number of neutrons, we subtract the atomic number from the atomic mass.
The atomic mass of tritium is approximately 3.016 amu (atomic mass units).
**Calculation**
To calculate the sum of neutrons and protons in tritium, we subtract the atomic number from the atomic mass:
Atomic Mass - Atomic Number = Neutrons
3.016 amu - 1 = 2.016 amu
Therefore, tritium has 2 neutrons.
**Sum of Neutrons and Protons**
To find the sum of neutrons and protons in tritium, we add the atomic number (protons) to the number of neutrons:
Protons + Neutrons = Sum
1 (proton) + 2 (neutrons) = 3
Therefore, the sum of the number of neutrons and protons in tritium is 3.
**Conclusion**
The correct answer is option C), which states that the sum of the number of neutrons and protons in tritium is 3. This is derived from the atomic mass of tritium (3.016 amu) and its atomic number (1), allowing us to calculate the number of neutrons (2) and the final sum (3).
Tritium is a radioactive isotope of hydrogen and has an atomic number of 1. It is denoted by the symbol T or ^3H. The atomic number represents the number of protons in an atom. To determine the sum of neutrons and protons in tritium, we need to know its atomic mass.
**Atomic Mass of Tritium**
The atomic mass of an atom is the sum of its protons and neutrons. Since tritium has an atomic number of 1, we know it has 1 proton. To find the number of neutrons, we subtract the atomic number from the atomic mass.
The atomic mass of tritium is approximately 3.016 amu (atomic mass units).
**Calculation**
To calculate the sum of neutrons and protons in tritium, we subtract the atomic number from the atomic mass:
Atomic Mass - Atomic Number = Neutrons
3.016 amu - 1 = 2.016 amu
Therefore, tritium has 2 neutrons.
**Sum of Neutrons and Protons**
To find the sum of neutrons and protons in tritium, we add the atomic number (protons) to the number of neutrons:
Protons + Neutrons = Sum
1 (proton) + 2 (neutrons) = 3
Therefore, the sum of the number of neutrons and protons in tritium is 3.
**Conclusion**
The correct answer is option C), which states that the sum of the number of neutrons and protons in tritium is 3. This is derived from the atomic mass of tritium (3.016 amu) and its atomic number (1), allowing us to calculate the number of neutrons (2) and the final sum (3).
Number of neutrons in Tritium are:- a)3
- b)2
- c)4
- d)1
Correct answer is option 'B'. Can you explain this answer?
Number of neutrons in Tritium are:
a)
3
b)
2
c)
4
d)
1
|
|
Raghav Bansal answered |
Sum of number of neutrons and protons represents mass number. Tritium 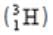 has 1 proton and 2 neutrons.
has 1 proton and 2 neutrons.
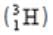 has 1 proton and 2 neutrons.
has 1 proton and 2 neutrons. Decomposition of H2O2 yields- a)H and O
- b)H2 and O2
- c)H2O and O2
- d)H+ and OH–
Correct answer is option 'C'. Can you explain this answer?
Decomposition of H2O2 yields
a)
H and O
b)
H2 and O2
c)
H2O and O2
d)
H+ and OH–
|
|
Neha Joshi answered |
2H2O2  2H2O + O2
2H2O + O2
The decomposition of hydrogen peroxide to produce oxygen and water. Laboratory method receiving of oxygen. This reaction takes place at a temperature of over 150°C or at room temperature and in the presence of a catalyst. In this reaction, the catalyst is can be sodium hydroxide, manganese(IV) oxide, platinum, copper.
 2H2O + O2
2H2O + O2The decomposition of hydrogen peroxide to produce oxygen and water. Laboratory method receiving of oxygen. This reaction takes place at a temperature of over 150°C or at room temperature and in the presence of a catalyst. In this reaction, the catalyst is can be sodium hydroxide, manganese(IV) oxide, platinum, copper.
Th e dielectric con stant of H2O is 80. The electrostatic force of attraction between Na+ and Cl– will be [1994]- a)reduced to 1/40 in water than in air
- b)reduced to 1/80 in water than in air
- c)will be increased to 80 in water than in air
- d)will remain unchanged.
Correct answer is option 'B'. Can you explain this answer?
Th e dielectric con stant of H2O is 80. The electrostatic force of attraction between Na+ and Cl– will be [1994]
a)
reduced to 1/40 in water than in air
b)
reduced to 1/80 in water than in air
c)
will be increased to 80 in water than in air
d)
will remain unchanged.

|
Krish Saha answered |
Electrostatic forces of attraction are reduced to 1/80th in water.
Temporary hardness It can be removed in boiling by precipitating- a)CaCO3.Mg(OH)2
- b)Mg(HCO3)2.CaCO3
- c)Mg(HCO3)2Ca(HCO3)2
- d)Ca(HCO3)2.Mg(OH)2
Correct answer is option 'A'. Can you explain this answer?
Temporary hardness It can be removed in boiling by precipitating
a)
CaCO3.Mg(OH)2
b)
Mg(HCO3)2.CaCO3
c)
Mg(HCO3)2Ca(HCO3)2
d)
Ca(HCO3)2.Mg(OH)2

|
Anisha Deshpande answered |
CaCO3.Mg(OH)2 can be precipitate out in order to remove temporary hardness.
Which of the following groups of ions makes the water hard ? [1994]- a)Sodium and bicarbonate
- b)Magnesium and chloride
- c)Potassium and sulphate
- d)Ammonium and chloride.
Correct answer is option 'B'. Can you explain this answer?
Which of the following groups of ions makes the water hard ? [1994]
a)
Sodium and bicarbonate
b)
Magnesium and chloride
c)
Potassium and sulphate
d)
Ammonium and chloride.

|
Palak Khanna answered |
Temporary hardness is due to presence of bicarbonates of calcium and magnesium and permanent hardness is due to the sulphates or chlorides of both of calcium and magnesium.
The volume strength of 1.5 NH2O2 solution is- a)4.8 L
- b)5.2 L [1996]
- c)8.4 L
- d)8.8 L
Correct answer is option 'C'. Can you explain this answer?
The volume strength of 1.5 NH2O2 solution is
a)
4.8 L
b)
5.2 L [1996]
c)
8.4 L
d)
8.8 L

|
Aman Sharma answered |
Volume strength = 5.6 × Normality = 5.6 × 1.5 = 8.4 L
Terrestrial hydrogen contains deuterium mostly in the form of:- a)SD
- b)MD
- c)NR
- d)HD
Correct answer is option 'D'. Can you explain this answer?
Terrestrial hydrogen contains deuterium mostly in the form of:
a)
SD
b)
MD
c)
NR
d)
HD
|
|
Himanshu Raj answered |
As we know that deuterium is present in very less amount than the hydrogen hence we can conclude from this that the deuterium will form hdnot even the surface of earth is state of it is also found outside the earth that is in the universe
Atoms like N, O and F in hydrides have lower boiling points than those of the subsequent group member hydrides. It is because of- a)shared electron pairs of N, O and F
- b)the magnitude of covalent bonding in their hydrides
- c)higher electronegativity of N, O and F
- d)higher electropositivity of N, O and F
Correct answer is option 'C'. Can you explain this answer?
Atoms like N, O and F in hydrides have lower boiling points than those of the subsequent group member hydrides. It is because of
a)
shared electron pairs of N, O and F
b)
the magnitude of covalent bonding in their hydrides
c)
higher electronegativity of N, O and F
d)
higher electropositivity of N, O and F

|
Aryan Dasgupta answered |
N,O and F are highly electronegative.
Tritium is an isotope of the element:- a)Chlorine
- b)Helium
- c)Sodium
- d)Hydrogen
Correct answer is option 'D'. Can you explain this answer?
Tritium is an isotope of the element:
a)
Chlorine
b)
Helium
c)
Sodium
d)
Hydrogen
|
|
Pooja Shah answered |
hydrogen, also known as hydrogen-3) is a radioactive isotope ofhydrogen. The nucleus of tritium (sometimes called a triton) contains one proton and two neutrons, whereas the nucleus of protium (by far the most abundant hydrogenisotope) contains one proton and no neutrons.
Water has maximum density at:- a)1°C
- b)0°C
- c)4K
- d)4°C
Correct answer is 'D'. Can you explain this answer?
Water has maximum density at:
a)
1°C
b)
0°C
c)
4K
d)
4°C
|
|
Mira Sharma answered |
Water has an especially notable irregular maximum density, which reaches a density peak at 3.98 degree C (approx. 4 deg C)(39.16 degree F).
When the ice melts to liquid water, the structure collapses and the density of the liquid increases. At temperatures well above freezing, the molecules move faster and get further apart. The density decreases as temperature increases. Thus, the density of water is a maximum at 4 degree C.
Some statements about heavy water are given below:
(a) Heavy water is used as a moderator in nuclear reactors.
(b) Heavy water is more associated than ordinary water.
(c) Heavy water is more effective solvent than ordinary water.
Which of the above statements are correct?- a)(a)and (c)
- b)(a) and (b) [2010]
- c) (a), (b) and (c)
- d)(b) and (c)
Correct answer is option 'B'. Can you explain this answer?
Some statements about heavy water are given below:
(a) Heavy water is used as a moderator in nuclear reactors.
(b) Heavy water is more associated than ordinary water.
(c) Heavy water is more effective solvent than ordinary water.
Which of the above statements are correct?
(a) Heavy water is used as a moderator in nuclear reactors.
(b) Heavy water is more associated than ordinary water.
(c) Heavy water is more effective solvent than ordinary water.
Which of the above statements are correct?
a)
(a)and (c)
b)
(a) and (b) [2010]
c)
(a), (b) and (c)
d)
(b) and (c)

|
Pankaj Banerjee answered |
∴ Correct choice : (b)
Reaction of granulated zinc with dil HCl results in formation of:- a)Dihydrogen
- b)Tritium
- c)Protium
- d)HD
Correct answer is option 'A'. Can you explain this answer?
Reaction of granulated zinc with dil HCl results in formation of:
a)
Dihydrogen
b)
Tritium
c)
Protium
d)
HD
|
|
Hansa Sharma answered |
Zn + 2HCl ------> ZnCl2 + H2 since it forms 2 moles of hydrogen it is a dihydrogen.
Consider the reactions(A) H2O2 + 2HI → I2 +2H2O
(B) HOCl + H2O2 → H3O + Cl - - + O2 Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen perioxide is ________.- a)an oxidising agent in (A) and reducing agent in (B)
- b)a reducing agent in both (A) and (B)
- c)an oxidising agent in both (A) and (B)
- d)a reducing agent in (A) and oxidising agent in (B)
Correct answer is option 'A'. Can you explain this answer?
Consider the reactions
(A) H2O2 + 2HI → I2 +2H2O
(B) HOCl + H2O2 → H3O + Cl - - + O2
(B) HOCl + H2O2 → H3O + Cl - - + O2
Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen perioxide is ________.
a)
an oxidising agent in (A) and reducing agent in (B)
b)
a reducing agent in both (A) and (B)
c)
an oxidising agent in both (A) and (B)
d)
a reducing agent in (A) and oxidising agent in (B)

|
Poulomi Datta answered |
H2O2 is an oxidizing agent in 1st reaction and Reducing agent in 2nd reaction.
Rocket fuel used in space research is- a)H2
- b)hydrogen peroxide
- c)H2O
- d)Lithium hydride
Correct answer is option 'A'. Can you explain this answer?
Rocket fuel used in space research is
a)
H2
b)
hydrogen peroxide
c)
H2O
d)
Lithium hydride

|
Subhankar Mukherjee answered |
Rocket Fuel is used in space research is H2
Do ice float on Water?- a)may be
- b)yes
- c)cannot say
- d)no
Correct answer is option 'B'. Can you explain this answer?
Do ice float on Water?
a)
may be
b)
yes
c)
cannot say
d)
no
|
|
Nandini Iyer answered |
Ice floats on water, this is because of the density of ice i.e. mass per unit volume (density of ice is 0.9167 g/cm3 and density of water is 1g/cm3) is lesser than that of water. This is the reason, ice floats over water.
NaH when added to water produces a large amount of energy. The hydride will be:- a)Interstitial hydride
- b)Covalent hydride
- c)Ionic hydride
- d)All of the above
Correct answer is option 'C'. Can you explain this answer?
NaH when added to water produces a large amount of energy. The hydride will be:
a)
Interstitial hydride
b)
Covalent hydride
c)
Ionic hydride
d)
All of the above
|
|
Rajat Patel answered |
Ionic hydrides are commonly known as saline hydrides or pseudohalides. These compounds form between hydrogen and the most active metals, especially with the alkali and alkaline-earth metals of group one and two elements. In this group, the hydrogen acts as the hydride ion (H−).
Of all the isotopes of hydrogen which one is highly radioactive:- a)HD
- b)Tritium
- c)Protium
- d)Deuterium
Correct answer is option 'B'. Can you explain this answer?
Of all the isotopes of hydrogen which one is highly radioactive:
a)
HD
b)
Tritium
c)
Protium
d)
Deuterium
|
|
Geetika Shah answered |
Tritium is the most stable radioisotope ofhydrogen. That is, of all radioactive isotopes of hydrogen, tritium is the leastradioactive. Scientists had created four other radioactive hydrogen isotopes, but these isotopes are very unstable and does not exist naturally.
Which one of the following pairs of substances on reaction will not evolve H2 gas? [1998]- a)Iron and H2SO4 (aqueous)
- b)Iron and steam
- c)Copper and HCl (aqueous)
- d)Sodium and ethyl alcohol
Correct answer is option 'C'. Can you explain this answer?
Which one of the following pairs of substances on reaction will not evolve H2 gas? [1998]
a)
Iron and H2SO4 (aqueous)
b)
Iron and steam
c)
Copper and HCl (aqueous)
d)
Sodium and ethyl alcohol

|
Ayush Choudhury answered |
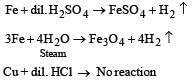
Copper does not evolve H2 from acid as it is below hydrogen in electro chemical series.
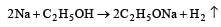
Hydrogen differs from alkali metals as it:- a)Has one electron in its valency shell
- b)Halides of hydrogen ionizes in aqueous solution
- c)Can form compounds with non-metals
- d)Does not possess metallic character
Correct answer is option 'D'. Can you explain this answer?
Hydrogen differs from alkali metals as it:
a)
Has one electron in its valency shell
b)
Halides of hydrogen ionizes in aqueous solution
c)
Can form compounds with non-metals
d)
Does not possess metallic character

|
Aditya Sengupta answered |
Hydrogen is a nonmetal and is placed above group in the periodic table because it has ns1 electron configuration like the alkali metals. However, it varies greatly from the alkali metals as it forms cations (H+) more reluctantly than the other alkali metals.
Which of the following statements are not true for hydrogen?- a)It can lose an electron to form a cation which can freely exist
- b)It cannot forms a large number of ionic compounds by losing an electrons.
- c)It has one electron in the outermost shell.
- d)It exists as diatomic molecule.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements are not true for hydrogen?
a)
It can lose an electron to form a cation which can freely exist
b)
It cannot forms a large number of ionic compounds by losing an electrons.
c)
It has one electron in the outermost shell.
d)
It exists as diatomic molecule.
|
|
Raghav Bansal answered |
The electronic configuration of the Hydrogen atom is 1s1.
It has one electron in the outermost shell. It exists as a diatomic molecule (H2) by sharing its' electron with other hydrogen atom.
It cannot form ionic compounds because it cannot donate its' electron and can only form covalent compounds by sharing the electron. It cannot lose an electron to form a cation because the hydrogen atom cannot exist after losing an electron.
H2O2 can be used as:- a)Bleaching agent
- b)Oxidizing agent
- c)Antiseptic
- d)All the above
Correct answer is option 'D'. Can you explain this answer?
H2O2 can be used as:
a)
Bleaching agent
b)
Oxidizing agent
c)
Antiseptic
d)
All the above

|
Ameya Pillai answered |
H2O2 can be used as:
There are multiple uses of H2O2, also known as hydrogen peroxide. It is a versatile compound with various applications. The correct answer to the question is option 'D', which means all of the above options are correct.
Bleaching agent:
Hydrogen peroxide is commonly used as a bleaching agent. It can remove stains and discoloration from fabrics, hair, and other materials. It is especially effective in removing tough stains like blood and wine. Its bleaching properties are due to its ability to break down into water and oxygen, which helps in oxidizing and removing the pigment or stain.
Oxidizing agent:
Hydrogen peroxide is a powerful oxidizing agent. It readily donates oxygen atoms, making it useful in various chemical reactions. It can oxidize metals, such as iron, copper, and silver, and is used in the production of certain chemicals and materials. It is also used in the treatment of wastewater and contaminated soils, where it helps in breaking down and oxidizing harmful compounds.
Antiseptic:
Hydrogen peroxide is widely used as an antiseptic to clean wounds and prevent infection. When it comes into contact with damaged tissues, it releases oxygen, which helps in killing bacteria, viruses, and fungi. It also helps in removing debris and promoting healing. However, it should be used with caution and in diluted form, as high concentrations can damage healthy tissues.
Other uses:
Apart from the above-mentioned uses, hydrogen peroxide has several other applications. It is used as a cleaning agent for household surfaces, such as countertops and bathroom fixtures. It is also used in the beauty industry for teeth whitening, mouthwash, and hair bleaching. Additionally, it is used in the manufacturing of various chemicals, as a rocket propellant, and in the food industry as a preservative.
In conclusion, hydrogen peroxide (H2O2) can be used as a bleaching agent, oxidizing agent, and antiseptic. Its versatility and properties make it a useful compound in various industries and applications.
There are multiple uses of H2O2, also known as hydrogen peroxide. It is a versatile compound with various applications. The correct answer to the question is option 'D', which means all of the above options are correct.
Bleaching agent:
Hydrogen peroxide is commonly used as a bleaching agent. It can remove stains and discoloration from fabrics, hair, and other materials. It is especially effective in removing tough stains like blood and wine. Its bleaching properties are due to its ability to break down into water and oxygen, which helps in oxidizing and removing the pigment or stain.
Oxidizing agent:
Hydrogen peroxide is a powerful oxidizing agent. It readily donates oxygen atoms, making it useful in various chemical reactions. It can oxidize metals, such as iron, copper, and silver, and is used in the production of certain chemicals and materials. It is also used in the treatment of wastewater and contaminated soils, where it helps in breaking down and oxidizing harmful compounds.
Antiseptic:
Hydrogen peroxide is widely used as an antiseptic to clean wounds and prevent infection. When it comes into contact with damaged tissues, it releases oxygen, which helps in killing bacteria, viruses, and fungi. It also helps in removing debris and promoting healing. However, it should be used with caution and in diluted form, as high concentrations can damage healthy tissues.
Other uses:
Apart from the above-mentioned uses, hydrogen peroxide has several other applications. It is used as a cleaning agent for household surfaces, such as countertops and bathroom fixtures. It is also used in the beauty industry for teeth whitening, mouthwash, and hair bleaching. Additionally, it is used in the manufacturing of various chemicals, as a rocket propellant, and in the food industry as a preservative.
In conclusion, hydrogen peroxide (H2O2) can be used as a bleaching agent, oxidizing agent, and antiseptic. Its versatility and properties make it a useful compound in various industries and applications.
Like alkali metals hydrogen also forms:- a)Optical isomers
- b)Homolytic cleavage
- c)Oxides,halides and sulphides
- d)Metallic bonding
Correct answer is option 'C'. Can you explain this answer?
Like alkali metals hydrogen also forms:
a)
Optical isomers
b)
Homolytic cleavage
c)
Oxides,halides and sulphides
d)
Metallic bonding
|
|
Om Desai answered |
Hydrogen has electronic configuration 1s Its electronic configuration is similar to the outer electronic configuration (ns) of alkali metals, which belong to the first group of the periodic table. Hydrogen therefore has resemblance to alkali metals, which lose one electron to form unipositive ions. Like alkali metals, hydrogen forms oxides, halides and sulphides.
Which of the following statements is correct?- a)Temporary hardness of water can be removed by boiling.
- b)Permanent hardness of water can be removed by boiling.
- c)Temporary and permanent hardness of water can be removed by boiling.
- d)Neither temporary nor permanent hardness of water can be removed by boiling.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements is correct?
a)
Temporary hardness of water can be removed by boiling.
b)
Permanent hardness of water can be removed by boiling.
c)
Temporary and permanent hardness of water can be removed by boiling.
d)
Neither temporary nor permanent hardness of water can be removed by boiling.
|
|
Neha Joshi answered |
Temporary hardness: It is due to the presence of soluble bicarbonates of calcium and magnesium. It can be removed by boiling. During boiling, the soluble Mg(HCO3)2 present in water is converted into insoluble Mg(OH)2 and Ca(HCO3)2 is changed to insoluble CaCO3 which settle at the bottom as precipitates. These precipitates can be removed by filtration. Filtrate thus obtained will be soft water.
Reactions:
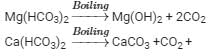
Reactions:

Element that is found abundantly in the universe and is the principal element of solar atmosphere is:- a)Helium
- b)Sodium
- c)Dihydrogen, H2
- d)Aluminium
Correct answer is option 'C'. Can you explain this answer?
Element that is found abundantly in the universe and is the principal element of solar atmosphere is:
a)
Helium
b)
Sodium
c)
Dihydrogen, H2
d)
Aluminium
|
|
Anjana Sharma answered |
Dihydrogen is the most abundant element in the universe (70% of the total mass of the universe) and is the principal element in the solar atmosphere. The giant planets Jupiter and Saturn consist mostly of hydrogen. However, due to its light nature, it is much less abundant (0.15% by mass) in the earth's atmosphere.
The reaction of H2O2 with H2S is an example of ........reaction
[1988]
- a)Addition
- b)Oxidation
- c)Reduction
- d)Redox
Correct answer is option 'D'. Can you explain this answer?
The reaction of H2O2 with H2S is an example of ........reaction
[1988]
a)
Addition
b)
Oxidation
c)
Reduction
d)
Redox

|
Rhea Sarkar answered |
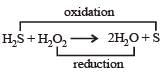
In this reaction H2S is oxidised to sulphur and H2O2 is reduced to H2O, hence this reaction show oxidation-reduction both i.e., redox reaction.
Which fluid compartment contains about 67% (by volume) of all body water?- a)intracellular fluid
- b)plasma
- c)lymph
- d)extracellular fluid
Correct answer is option 'A'. Can you explain this answer?
Which fluid compartment contains about 67% (by volume) of all body water?
a)
intracellular fluid
b)
plasma
c)
lymph
d)
extracellular fluid
|
|
Vijay Bansal answered |
Intracellular fluid (2/3 of body water) is fluid contained within cells. In a 72-kg body containing 40 litres of fluid, about 25 litres is intracellular, which amounts to 62.5%. Jackson's texts states 70% of body fluid is intracellular.
Calculate the strength of 5 volume H2O2 solution. - a)2.5% solution
- b)15 g/L solution
- c)10 g/L solution
- d)3.5% solution
Correct answer is option 'B'. Can you explain this answer?
Calculate the strength of 5 volume H2O2 solution.
a)
2.5% solution
b)
15 g/L solution
c)
10 g/L solution
d)
3.5% solution
|
|
Lavanya Menon answered |
Volume strength = 11.2 x M So M = 5/11.2 = 0.446 Strength (g/L ) = 0.446 x 34 = 15.17 g/L
The physical properties of isotopes differ due to:- a)Similar chemical properties
- b)Large physical differences
- c)Large mass differences
- d)Large rate of reaction
Correct answer is option 'C'. Can you explain this answer?
The physical properties of isotopes differ due to:
a)
Similar chemical properties
b)
Large physical differences
c)
Large mass differences
d)
Large rate of reaction
|
|
Nandini Patel answered |
The physical properties of isotopes of the same element are not identical because the atomic mass and mass number differ. This affects physical properties such as density and temperature of change of state, eg. boiling and melting point.
When a substance A reacts with water it produces a combustible gas B and a solution of substance C in water. When another substance D reacts with this solution of C, it also produces the same gas B on warming but D can produce gas B on reaction with dilute sulphuric acid at room temperature. A imparts a deep golden yellow colour to a smokeless flame of Bunsen burner. A, B, C and D respectively are [1998]- a)Na , H2, NaOH, Zn
- b)K, H2, KOH, Al
- c)Ca, H2, Ca(OH)2, Sn
- d)CaC2, C2H2, Ca(OH)2, Fe
Correct answer is option 'A'. Can you explain this answer?
When a substance A reacts with water it produces a combustible gas B and a solution of substance C in water. When another substance D reacts with this solution of C, it also produces the same gas B on warming but D can produce gas B on reaction with dilute sulphuric acid at room temperature. A imparts a deep golden yellow colour to a smokeless flame of Bunsen burner. A, B, C and D respectively are [1998]
a)
Na , H2, NaOH, Zn
b)
K, H2, KOH, Al
c)
Ca, H2, Ca(OH)2, Sn
d)
CaC2, C2H2, Ca(OH)2, Fe

|
Abhishek Choudhary answered |
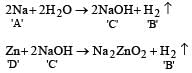
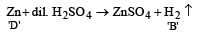
Na produces golden yellow colour with smokeless flame of Bunsen burner.
Hydrides are classified as:- a)Non-ionic,non-covalent and non-metallic
- b)Non-covalent,non-molecular and stoichiometric
- c)Ionic,covalent and metallic
- d)Non-molecular,non-metallic and stoichiometric
Correct answer is option 'C'. Can you explain this answer?
Hydrides are classified as:
a)
Non-ionic,non-covalent and non-metallic
b)
Non-covalent,non-molecular and stoichiometric
c)
Ionic,covalent and metallic
d)
Non-molecular,non-metallic and stoichiometric

|
Akshay Sharma answered |
Hydrides are classified into three major groups, depending on what elements the hydrogen bonds to. The three major groups are covalent, ionic, and metallic hydrides. Formally, hydride is known as the negative ion of a hydrogen, H-, also called a hydride ion.
Which of the following is not an isotope of hydrogen?- a)helium
- b)protium
- c)deuterium
- d)tritium
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not an isotope of hydrogen?
a)
helium
b)
protium
c)
deuterium
d)
tritium

|
Milan Datta answered |
Answer:
Introduction:
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Hydrogen is the lightest and simplest element in the periodic table, with only one proton and one electron. However, there are three isotopes of hydrogen that differ in their number of neutrons.
Explanation:
The three isotopes of hydrogen are protium, deuterium, and tritium. Let's discuss each isotope in detail:
1. Protium (Symbol: H-1):
Protium is the most common and abundant isotope of hydrogen, making up about 99.985% of naturally occurring hydrogen. It consists of one proton and no neutrons. Protium is the simplest form of hydrogen, and its nucleus contains only a single proton.
2. Deuterium (Symbol: H-2):
Deuterium is a stable isotope of hydrogen that is relatively rare, making up about 0.015% of naturally occurring hydrogen. It consists of one proton and one neutron in its nucleus. Deuterium is often used as a tracer in chemical and biological research.
3. Tritium (Symbol: H-3):
Tritium is a radioactive isotope of hydrogen, with one proton and two neutrons. It is highly unstable and decays over time with a half-life of about 12.3 years. Tritium is used in a variety of applications, including as a tracer in research and in self-luminous exit signs.
Conclusion:
Among the given options, helium (He) is not an isotope of hydrogen. Helium is a completely different element with two protons and two neutrons in its nucleus. It is the second lightest element in the periodic table and is a noble gas.
Introduction:
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Hydrogen is the lightest and simplest element in the periodic table, with only one proton and one electron. However, there are three isotopes of hydrogen that differ in their number of neutrons.
Explanation:
The three isotopes of hydrogen are protium, deuterium, and tritium. Let's discuss each isotope in detail:
1. Protium (Symbol: H-1):
Protium is the most common and abundant isotope of hydrogen, making up about 99.985% of naturally occurring hydrogen. It consists of one proton and no neutrons. Protium is the simplest form of hydrogen, and its nucleus contains only a single proton.
2. Deuterium (Symbol: H-2):
Deuterium is a stable isotope of hydrogen that is relatively rare, making up about 0.015% of naturally occurring hydrogen. It consists of one proton and one neutron in its nucleus. Deuterium is often used as a tracer in chemical and biological research.
3. Tritium (Symbol: H-3):
Tritium is a radioactive isotope of hydrogen, with one proton and two neutrons. It is highly unstable and decays over time with a half-life of about 12.3 years. Tritium is used in a variety of applications, including as a tracer in research and in self-luminous exit signs.
Conclusion:
Among the given options, helium (He) is not an isotope of hydrogen. Helium is a completely different element with two protons and two neutrons in its nucleus. It is the second lightest element in the periodic table and is a noble gas.
90% of hydrogen peroxide is used as fuel in ______________- a)electricity
- b)submarines
- c)bike
- d)cycle
Correct answer is option 'B'. Can you explain this answer?
90% of hydrogen peroxide is used as fuel in ______________
a)
electricity
b)
submarines
c)
bike
d)
cycle
|
|
Neha Sharma answered |
Hydrogen peroxide has many uses one of the most important uses of hydrogen peroxide is that it acts as a source of power i.e. 90% of hydrogen peroxide is used as fuel and submarines, rockets and helicopters.
What does hydrogen peroxide liberate from potassium iodide?- a)Nitrogen
- b)Potassium
- c)Iodine
- d)Hydrogen
Correct answer is option 'C'. Can you explain this answer?
What does hydrogen peroxide liberate from potassium iodide?
a)
Nitrogen
b)
Potassium
c)
Iodine
d)
Hydrogen
|
|
Hansa Sharma answered |
When 2 moles of potassium iodide are reacted with 1 mole of hydrogen peroxide, 2 moles of potassium hydroxide and one mole of iodine is liberated. That means hydrogen peroxide liberates iodine from acidified potassium iodide.
What is 30% of hydrogen peroxide called?- a)Iodine
- b)Perhydrol
- c)Hydro
- d)Chlorine
Correct answer is option 'B'. Can you explain this answer?
What is 30% of hydrogen peroxide called?
a)
Iodine
b)
Perhydrol
c)
Hydro
d)
Chlorine

|
Sounak Chaudhary answered |
30% hydrogen peroxide is called perhydrol.
Hydrogen peroxide is a chemical compound with the formula H2O2. It is a pale blue liquid that appears colorless in a dilute solution and is a powerful oxidizer.
Perhydrol is the name given to hydrogen peroxide solutions that have a concentration of 30%.
Hydrogen peroxide is commonly available in different concentrations, ranging from 3% to 90%. The concentration refers to the amount of hydrogen peroxide present in the solution compared to the total volume. For example, a 3% hydrogen peroxide solution contains 3 grams of hydrogen peroxide per 100 milliliters of solution.
Perhydrol, specifically the 30% concentration, is often used for various purposes, including:
- Bleaching and disinfecting agents: Perhydrol is commonly used as a bleaching agent for textiles, hair, and teeth. It can also act as a disinfectant for surfaces, wounds, and medical equipment.
- Industrial applications: Perhydrol is utilized in various industrial processes, such as pulp and paper bleaching, wastewater treatment, and the production of chemicals like sodium percarbonate and sodium perborate.
- Laboratory use: Perhydrol is used in laboratories for cleaning glassware, sterilizing equipment, and as a reagent in chemical reactions.
It is important to handle perhydrol with care due to its corrosive and oxidizing properties. It can cause skin and eye irritation, and it should not be ingested. Perhydrol can react violently with certain substances, such as combustible materials, reducing agents, and metals.
In conclusion, 30% hydrogen peroxide is commonly referred to as perhydrol. It is a versatile compound used in various applications, including bleaching, disinfection, and industrial processes. Due to its corrosive nature, caution should be exercised when handling perhydrol.
Hydrogen peroxide is a chemical compound with the formula H2O2. It is a pale blue liquid that appears colorless in a dilute solution and is a powerful oxidizer.
Perhydrol is the name given to hydrogen peroxide solutions that have a concentration of 30%.
Hydrogen peroxide is commonly available in different concentrations, ranging from 3% to 90%. The concentration refers to the amount of hydrogen peroxide present in the solution compared to the total volume. For example, a 3% hydrogen peroxide solution contains 3 grams of hydrogen peroxide per 100 milliliters of solution.
Perhydrol, specifically the 30% concentration, is often used for various purposes, including:
- Bleaching and disinfecting agents: Perhydrol is commonly used as a bleaching agent for textiles, hair, and teeth. It can also act as a disinfectant for surfaces, wounds, and medical equipment.
- Industrial applications: Perhydrol is utilized in various industrial processes, such as pulp and paper bleaching, wastewater treatment, and the production of chemicals like sodium percarbonate and sodium perborate.
- Laboratory use: Perhydrol is used in laboratories for cleaning glassware, sterilizing equipment, and as a reagent in chemical reactions.
It is important to handle perhydrol with care due to its corrosive and oxidizing properties. It can cause skin and eye irritation, and it should not be ingested. Perhydrol can react violently with certain substances, such as combustible materials, reducing agents, and metals.
In conclusion, 30% hydrogen peroxide is commonly referred to as perhydrol. It is a versatile compound used in various applications, including bleaching, disinfection, and industrial processes. Due to its corrosive nature, caution should be exercised when handling perhydrol.
At high electric discharge hydrogen and oxygen combine to form _____________- a)acid
- b)water
- c)hydrogen peroxide
- d)salt
Correct answer is option 'C'. Can you explain this answer?
At high electric discharge hydrogen and oxygen combine to form _____________
a)
acid
b)
water
c)
hydrogen peroxide
d)
salt
|
|
Akash Chakraborty answered |
Formation of Hydrogen Peroxide:
Hydrogen and oxygen combine to form hydrogen peroxide at high electric discharge. This process involves the following steps:
- Ionization of Hydrogen and Oxygen: When high electric discharge is applied, hydrogen and oxygen molecules become ionized, breaking into their constituent atoms.
- Formation of Free Radicals: The ionized atoms of hydrogen and oxygen then form free radicals, which are highly reactive species due to the presence of unpaired electrons.
- Combination of Hydrogen and Oxygen: The free radicals of hydrogen and oxygen combine to form hydrogen peroxide (H2O2) molecule.
- Chemical Equation: The overall chemical equation for the formation of hydrogen peroxide can be represented as: 2H + 2O -> 2H2O2
- Properties of Hydrogen Peroxide: Hydrogen peroxide is a colorless and odorless liquid that is slightly more viscous than water. It is a powerful oxidizing agent and has various uses in industries and laboratories.
In conclusion, at high electric discharge, hydrogen and oxygen react to form hydrogen peroxide through a series of ionization, radical formation, and combination reactions. This process is crucial for the production of hydrogen peroxide, an important chemical compound with diverse applications.
Which of the following option is true regarding the boiling point of hydrogen isotopes?- a)all three isotopes have an equal boiling point
- b)deuterium has a greater boiling point than tritium
- c)tritium has a greater boiling point than that of protium
- d)protium has a higher boiling point than that of tritium
Correct answer is option 'C'. Can you explain this answer?
Which of the following option is true regarding the boiling point of hydrogen isotopes?
a)
all three isotopes have an equal boiling point
b)
deuterium has a greater boiling point than tritium
c)
tritium has a greater boiling point than that of protium
d)
protium has a higher boiling point than that of tritium
|
|
Preeti Iyer answered |
The boiling points of tritium are greater than that of deuterium and that is greater than that of hydrogen. The boiling points in kelvin of hydrogen, deuterium, and tritium are respectively 20.39, 23.67 and 25.
What is the temperature required in presence of molybdenum and iron for nitrogen to combine with hydrogen?- a)123 Kelvin
- b)273 Kelvin
- c)673 Kelvin
- d)473 Kelvin
Correct answer is option 'C'. Can you explain this answer?
What is the temperature required in presence of molybdenum and iron for nitrogen to combine with hydrogen?
a)
123 Kelvin
b)
273 Kelvin
c)
673 Kelvin
d)
473 Kelvin
|
|
Geetika Shah answered |
At 673 Kelvin and 200 atmospheric pressure, one mole of nitrogen combines with three moles of hydrogen in presence of iron and molybdenum in order to form 2 moles of ammonia and the enthalpy change is 92.6 KJ per Mol, the enthalpy here change is negative.
At its melting pointice is lighter than water because [1992]- a)H2O molecules are more closely packed in solid state
- b)Ice crystals have hollow hexagonal arrangement of H2O molecules.
- c)On melting of ice the H2O molecule shrinks in size
- d)Ice froms mostly heavy water on first melting.
Correct answer is option 'B'. Can you explain this answer?
At its melting pointice is lighter than water because [1992]
a)
H2O molecules are more closely packed in solid state
b)
Ice crystals have hollow hexagonal arrangement of H2O molecules.
c)
On melting of ice the H2O molecule shrinks in size
d)
Ice froms mostly heavy water on first melting.
|
|
Rohan Singh answered |
In the structure of ice each molecule of H2O is surrounded by three H2O molecules in hexagonal honey comb manner. On the other in water, each molecule is surrounded by four neigh bouring molecules randomly which results an open cage like structure. As a result there are a number of ‘hole’ or open spaces. In such a structure lesser number of molecules are packed per ml. When ice melts a large no. of hydrogen bonds are broken. The molecules therefore move into the holes or open spaces and come closer to each other than they were in solid state. This result sharp increase in the density. Therefore ice has lower density than water.
Heavy hydrogen is also called- a)dihydrogen
- b)tritium
- c)protium
- d)deuterium
Correct answer is option 'D'. Can you explain this answer?
Heavy hydrogen is also called
a)
dihydrogen
b)
tritium
c)
protium
d)
deuterium

|
Sushant Khanna answered |
Heavy hydrogen is deuterium.
One of the following is a non-stoichiometric hydride- a)Covalent hydrides
- b)Ionic hydrides
- c)Metallic hydrides
- d)molecular hydrides
Correct answer is option 'C'. Can you explain this answer?
One of the following is a non-stoichiometric hydride
a)
Covalent hydrides
b)
Ionic hydrides
c)
Metallic hydrides
d)
molecular hydrides

|
Nishanth Verma answered |
Metallic hydrides are non stoichiometric hydride.
Chapter doubts & questions for Hydrogen - Inorganic Chemistry for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Hydrogen - Inorganic Chemistry for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily