All Exams >
NEET >
Inorganic Chemistry for NEET >
All Questions
All questions of s- block Elements for NEET Exam
The formula of bleaching powder is:
- a)CaSO4.2H2O
- b)HCl
- c)CaOCl2
- d)ZnSO4
Correct answer is option 'C'. Can you explain this answer?
The formula of bleaching powder is:
a)
CaSO4.2H2O
b)
HCl
c)
CaOCl2
d)
ZnSO4
|
|
Raghav Bansal answered |
The chemical name of bleaching powder is calcium hypochlorite.it is manufactured by the action of chlorine on slaked lime (Ca(OH).
2Ca(OH)2+ 2Cl2→CaOCl2+ 2H2O+CaCl2
2Ca(OH)2+ 2Cl2→CaOCl2+ 2H2O+CaCl2
Milk of lime reacts with chlorine to produce:- a)Bleaching powder
- b)Quick lime
- c)Gypsum
- d)Slaked lime
Correct answer is option 'A'. Can you explain this answer?
Milk of lime reacts with chlorine to produce:
a)
Bleaching powder
b)
Quick lime
c)
Gypsum
d)
Slaked lime
|
|
Anjali Iyer answered |
Lime reacts readily with water to produce slaked lime, which is the chemical compound calcium hydroxide. A considerable amount of heat energy is released during this reaction. Slaked lime reacts with chlorine gas to produce the bleaching agent calcium hypochlorite – a common form of 'swimming pool' chlorine.
What is the oxidation state of K in KO2?- a)+2
- b)+4
- c)+3
- d)1.0
Correct answer is option 'D'. Can you explain this answer?
What is the oxidation state of K in KO2?
a)
+2
b)
+4
c)
+3
d)
1.0
|
|
Krishna Iyer answered |
Here KO2 is in superoxide form hence O.S. of O is -1/2 for each oxygen atom. Hence 2*1/2= 1, therefore k has +1 O.S.
Some of the Group 2 metal halides are covalent and soluble in organic solvents. Among the following metal halides, the one which is soluble in ethanol is - a)MgCl2
- b)SrCl2
- c)CaCl2
- d)BeCl2
Correct answer is option 'D'. Can you explain this answer?
Some of the Group 2 metal halides are covalent and soluble in organic solvents. Among the following metal halides, the one which is soluble in ethanol is
a)
MgCl2
b)
SrCl2
c)
CaCl2
d)
BeCl2
|
|
Mira Sharma answered |
Due to small size, high electronegativity and high ionization enthalpy of Be, BeCl2 is covalent and hence most soluble in organic solvents such as ethanol.
Passing carbon dioxide through slaked lime gives:- a)CaSO4
- b)CaCO3
- c)CaO
- d)Ca(OH)2
Correct answer is option 'B'. Can you explain this answer?
Passing carbon dioxide through slaked lime gives:
a)
CaSO4
b)
CaCO3
c)
CaO
d)
Ca(OH)2
|
|
Geetika Shah answered |
If CO2 is passed through slaked lime(calcium hydroxide Ca(OH)2), it gives out calcium carbonate (CaCO3) also called marble/limestone.
Reaction: CO2 + Ca(OH)2→ CaCO3+ H2O
Bones and teeth contains:- a)Na
- b)K
- c)Mg
- d)Ca
Correct answer is option 'D'. Can you explain this answer?
Bones and teeth contains:
a)
Na
b)
K
c)
Mg
d)
Ca
|
|
Vijay Bansal answered |
Teeth are composed of calcium, phosphorus, and other minerals. Bones contain calcium, phosphorus, sodium and other minerals, but mostly consist of the protein collagen. Collagen is a living, growing tissue that gives bones their a flexible framework that allows them to withstand pressure.
What is the oxidation state of Rb in RbO2?- a)-2
- b)+1
- c)-1
- d)+2
Correct answer is option 'B'. Can you explain this answer?
What is the oxidation state of Rb in RbO2?
a)
-2
b)
+1
c)
-1
d)
+2
|
|
Ishani Pillai answered |
Determining the Oxidation State of Rb in RbO2
Oxidation state or oxidation number refers to the charge that an atom would have if the electron pairs in a bond were assigned to the more electronegative atom. In RbO2, we can determine the oxidation state of Rb by following these steps:
Step 1: Identify the oxidation state of oxygen (O). Oxygen typically has an oxidation state of -2 in most compounds.
Step 2: Assign the oxidation state of Rb as x.
Step 3: Determine the total charge of the compound. Since RbO2 is a neutral compound, the sum of the oxidation states of all the atoms in the compound must be equal to zero.
Step 4: Apply the rule of charge balance to calculate the oxidation state of Rb. In RbO2, there are two oxygen atoms, each with an oxidation state of -2. Therefore, the total charge contributed by the oxygen atoms is -4.
-2 (oxidation state of O) x 2 (number of O atoms) = -4
To balance the charge, the oxidation state of Rb must be +4.
x + (-4) = 0
x = +4
Therefore, the oxidation state of Rb in RbO2 is +4, which is option B.
Oxidation state or oxidation number refers to the charge that an atom would have if the electron pairs in a bond were assigned to the more electronegative atom. In RbO2, we can determine the oxidation state of Rb by following these steps:
Step 1: Identify the oxidation state of oxygen (O). Oxygen typically has an oxidation state of -2 in most compounds.
Step 2: Assign the oxidation state of Rb as x.
Step 3: Determine the total charge of the compound. Since RbO2 is a neutral compound, the sum of the oxidation states of all the atoms in the compound must be equal to zero.
Step 4: Apply the rule of charge balance to calculate the oxidation state of Rb. In RbO2, there are two oxygen atoms, each with an oxidation state of -2. Therefore, the total charge contributed by the oxygen atoms is -4.
-2 (oxidation state of O) x 2 (number of O atoms) = -4
To balance the charge, the oxidation state of Rb must be +4.
x + (-4) = 0
x = +4
Therefore, the oxidation state of Rb in RbO2 is +4, which is option B.
Alkaline earth metals combine with halogens to form:- a)MX3
- b)MX1
- c)MX
- d)MX2
Correct answer is option 'D'. Can you explain this answer?
Alkaline earth metals combine with halogens to form:
a)
MX3
b)
MX1
c)
MX
d)
MX2
|
|
Geetika Shah answered |
Alkaline earth metals have valency +2. So on reaction with halogen, they form compounds MX2.
Choose the correct order of increase in electropositivity of alkali earth metals in the options given below
- a)Be2+<Mg2+<Ca2+<Sr2+<Ba2+
- b)Sr2+ > Ba2+ < Be2+ < Mg2+ < Sr2+
- c)Ca2+ > Sr2+ > Ba2+ < Be2+ < Mg2+
- d)Mg2+ > Ca2+ > Sr2+> Ba2+ < Be2+
Correct answer is option 'A'. Can you explain this answer?
Choose the correct order of increase in electropositivity of alkali earth metals in the options given below
a)
Be2+<Mg2+<Ca2+<Sr2+<Ba2+
b)
Sr2+ > Ba2+ < Be2+ < Mg2+ < Sr2+
c)
Ca2+ > Sr2+ > Ba2+ < Be2+ < Mg2+
d)
Mg2+ > Ca2+ > Sr2+> Ba2+ < Be2+
|
|
Suresh Iyer answered |
Correct Answer : a
Explanation : Atomic no. of Be, Mg, Ca, Sr, Ba are 4, 12,20,38,56 respectively.
On moving down the group, the effective nuclear charge on the outermost electrons decreases. Hence Ba can readily lose electrons and will be the most electropositive element among the alkaline earth metals.
Sodium and potassium belong to the category of- a)transition elements
- b)noble gases
- c)alkaline earth metals.
- d)alkali metals.
Correct answer is option 'D'. Can you explain this answer?
Sodium and potassium belong to the category of
a)
transition elements
b)
noble gases
c)
alkaline earth metals.
d)
alkali metals.
|
|
Preeti Iyer answered |
Na and K have ns1 configuration so they are alkali metal.
In the periodic table the element with atomic number 38 belongs to:- a)Period III and group IV
- b)Period IV and group IV
- c)Period V and group II
- d)Period IV and group II
Correct answer is option 'C'. Can you explain this answer?
In the periodic table the element with atomic number 38 belongs to:
a)
Period III and group IV
b)
Period IV and group IV
c)
Period V and group II
d)
Period IV and group II
|
|
Vijay Bansal answered |
The answer is very simple, I just calculated it, nothing else. For that I firstly subtracted 18 from it and then 8 and again 8, I got 4 and i very well knew that the element having atomic number 4 belong to the 2 group of the periodic table.I got the group but for the period i calculated how many times i subtracted any number from the given atomic no.,i got 3 and i added this 3 to the period of the element of atomic number 4 i.e. 2 i.e.2+3=5. So finally i got 5.Hence i concluded that the element is of the 2 nd group and 5 th period.
Now the question must be arising in your mind that why i subtracted 18 and then 8 and again 8.Let me tell you that.I knew that the difference between the atomic numbers of the 2nd period and 3rd period is 8 and similarly 3rd & 4th-also 8 , 4th & 5th-18,5th & 6th-18 only for first three groups then 32,6th &7th-again 32.
Now why only subtracted 18 from 38 ,why not 8 or 32?Here the answer is also very simple. Now if you get any number between 1 and 10 to tell its period and group you can say it orally.But the main juice is after 10.If the number comes between then 10 and 20 you easily have to subtract 8 and then tell the answer(but remember you have to 2 times 8 from element having atomic number 19 or 20).Now between 21 to 57 you should subtract 18 and then 2 times 8.Now after 57 you first have to subtract 32 then 18 and then 2 times 8.
This is my way , how i do this type of problem sometimes.
This way is not applicable for the F block.But for this block you should remember that all the elements are in 3rd group and if the atomic number of the required element is between 57 and 71 then it would be of 6th period and if it is between 89 to 103 it would be of 7th period.
Aqueous solution of which of the following compounds is used to detect carbon dioxide:- a)Mg(OH)2
- b)Sr(OH)2
- c)Ca(OH)2
- d)Be(OH)2
Correct answer is option 'C'. Can you explain this answer?
Aqueous solution of which of the following compounds is used to detect carbon dioxide:
a)
Mg(OH)2
b)
Sr(OH)2
c)
Ca(OH)2
d)
Be(OH)
2
|
|
Lavanya Menon answered |
On passing CO2 gas, Ca(OH)2 becomes CaCO3 (milky white ppt.) which proves the presence of CO2.
Be and Al form chlorides which are:- a)Arrhenius acids
- b)Bronsted Lowry acids
- c)Lewis acids
- d)Lewis bases
Correct answer is option 'C'. Can you explain this answer?
Be and Al form chlorides which are:
a)
Arrhenius acids
b)
Bronsted Lowry acids
c)
Lewis acids
d)
Lewis bases
|
|
Krishna Iyer answered |
Be and Al forms compound with Cl as BeCl
Li shows difference in properties than rest of the members of its group but shows similarities with:- a)Mg
- b)Al
- c)Ca
- d)Be
Correct answer is option 'A'. Can you explain this answer?
Li shows difference in properties than rest of the members of its group but shows similarities with:
a)
Mg
b)
Al
c)
Ca
d)
Be
|
|
Krishna Iyer answered |
Due to diagonal relationship, Li resembles Mg.
Alkaline earth metals show large size of the atoms. Therefore, they have- a)low hydration enthalpies
- b)low electopositivity
- c)low ionization enthalpies
- d)low nuclear charge
Correct answer is option 'C'. Can you explain this answer?
Alkaline earth metals show large size of the atoms. Therefore, they have
a)
low hydration enthalpies
b)
low electopositivity
c)
low ionization enthalpies
d)
low nuclear charge
|
|
Rohan Singh answered |
Alkaline Earth Metals Periodic Table. This group comprises of beryllium (4Be), magnesium (12Mg), calcium (20Ca), strontium (38Sr), barium (56Ba) and radium (88Ra).Just like alkali metals, alkaline earth metals properties show reactive nature and do not exist in the free state.
The sequence of ioinic mobility in aqueous solution is : [2008]- a)K+ > Na+ > Rb+ > Cs+
- b)Cs+ > Rb+ > K+ > Na+
- c)Rb+ > K+ > Cs+ > Na+
- d)Na+ > K+ > Rb+ > Cs+
Correct answer is option 'B'. Can you explain this answer?
The sequence of ioinic mobility in aqueous solution is : [2008]
a)
K+ > Na+ > Rb+ > Cs+
b)
Cs+ > Rb+ > K+ > Na+
c)
Rb+ > K+ > Cs+ > Na+
d)
Na+ > K+ > Rb+ > Cs+

|
Rohan Unni answered |
Smaller the ion more is its ionic mobility in aqueous solution. Ionic radii of the given alkali metals is in the order Na+ < K+ < Rb+ < Cs+ and thus expected ionic mobility will be in the order Cs+ < Rb+ < K+ < Na+. However due to high degree of solvation (or hydration) because of lower size or high charge density, the hydrated ion size follows the order Cs+ < Rb+ < K+ < Na+ and thus conductivity order is Cs+ > Rb+ > K+ > Na+ i.e. option (b) is correct answer.
What is washing soda?- a)Sodium Bicarbonate
- b)Sodium Chloride
- c)Sodium Hydroxide
- d)Sodium Carbonate
Correct answer is option 'D'. Can you explain this answer?
What is washing soda?
a)
Sodium Bicarbonate
b)
Sodium Chloride
c)
Sodium Hydroxide
d)
Sodium Carbonate
|
|
Rajeev Saxena answered |
Sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate) is the water-soluble sodium salt of carbonic acid.
It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic (absorbs moisture from the air). It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener.
Cs reacts with chlorine to form:- a)CsCl2
- b)Cs2Cl2
- c)CsCl
- d)CsCl3
Correct answer is option 'C'. Can you explain this answer?
Cs reacts with chlorine to form:
a)
CsCl2
b)
Cs2Cl2
c)
CsCl
d)
CsCl3

|
Aashika Packiaselvam answered |
It forms Cesium Chloride similar to table salt. So the answer is CsCl.
The correct order of second ionisation potentials (IP) of Ca, Ba and K is -- a)K > Ca > Ba
- b)Ba > Ca > K
- c)K > Ba > Ca
- d)K = Ba = Ca
Correct answer is option 'A'. Can you explain this answer?
The correct order of second ionisation potentials (IP) of Ca, Ba and K is -
a)
K > Ca > Ba
b)
Ba > Ca > K
c)
K > Ba > Ca
d)
K = Ba = Ca

|
Subhankar Mukherjee answered |
K [Ar] 4s1 → second ionisation is extremely difficult.
Ca [Ar] 4s2
Ba [Xe] 5S2
Ca [Ar] 4s2
Ba [Xe] 5S2
Which of the following statement is false ? [1994]- a)Strontium decomposes water readily than beryllium
- b)Barium carbonate melts at a higher temperature than calcium carbonate
- c)Barium hydroxide is more soluble in water than magnesium hydroxide
- d)Beryllium hydroxide is more basic than barium hydroxide.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is false ? [1994]
a)
Strontium decomposes water readily than beryllium
b)
Barium carbonate melts at a higher temperature than calcium carbonate
c)
Barium hydroxide is more soluble in water than magnesium hydroxide
d)
Beryllium hydroxide is more basic than barium hydroxide.

|
Pankaj Banerjee answered |
Be(OH)2 is amphoteric, but the hydroxides of other alkaline earth metals are basic. The basic strength increases gradually.
Alkaline earth metals show:- a)Variable valency
- b)Divalency
- c)Monovalency
- d)Zero valency
Correct answer is option 'B'. Can you explain this answer?
Alkaline earth metals show:
a)
Variable valency
b)
Divalency
c)
Monovalency
d)
Zero valency

|
Syed Hussain answered |
The alkaline earth metals are all of theelements in the second column (column 2A) of the periodic table. This group includes beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra). Alkaline earth metalshave only two electrons in their outermost electron layer.
Heating limestone at a temperature of 1070K we get:- a)Slaked lime
- b)Quick Lime
- c)Plaster of Paris
- d)CaCO3
Correct answer is option 'B'. Can you explain this answer?
Heating limestone at a temperature of 1070K we get:
a)
Slaked lime
b)
Quick Lime
c)
Plaster of Paris
d)
CaCO3
|
|
Anjana Sharma answered |
Heating limestone at a temperature of 1070K we get quick lime i.e CaO.
The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongst fluorides of alkali metals,the lowest solubility of LiF in water is due to- a)High lattice enthalpy
- b)Ionic nature of lithium fluoride
- c)High hydration enthalpy for lithium ion.
- d)Low ionisation enthalpy of lithium atom
Correct answer is option 'A'. Can you explain this answer?
The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongst fluorides of alkali metals,the lowest solubility of LiF in water is due to
a)
High lattice enthalpy
b)
Ionic nature of lithium fluoride
c)
High hydration enthalpy for lithium ion.
d)
Low ionisation enthalpy of lithium atom
|
|
Om Desai answered |
LiF has very high lattice energy which cannot be compensated by Hydration Energy.
In which of the following the hydration energy is higher than the lattice energy? [2007]- a)MgSO4
- b)RaSO4
- c)SrSO4
- d)BaSO4
Correct answer is option 'A'. Can you explain this answer?
In which of the following the hydration energy is higher than the lattice energy? [2007]
a)
MgSO4
b)
RaSO4
c)
SrSO4
d)
BaSO4

|
Pankaj Banerjee answered |
The solubility of sulphates of alkaline earth metals decreases as we move down the group from Be to Ba due to the reason that ionic size increases down the group. The lattice energy remains constant because sulphate ion is so large, so that small change in cationic sizes do not make any difference.
Thus the order:
Thus the order:
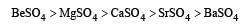
Identify the correct statement [1995]- a)gypsum is obtained by heating plaster of Paris
- b)plaster of Paris can be obtained by hydration of gypsum
- c)plaster of par is is obtain ed by par tial oxidation of gypsum
- d)gypsum contains a lower percentage of calcium than plaster of Paris
Correct answer is option 'D'. Can you explain this answer?
Identify the correct statement [1995]
a)
gypsum is obtained by heating plaster of Paris
b)
plaster of Paris can be obtained by hydration of gypsum
c)
plaster of par is is obtain ed by par tial oxidation of gypsum
d)
gypsum contains a lower percentage of calcium than plaster of Paris

|
Rajeev Sharma answered |
Gypsum is CaSO4. 2H2O and plaster of Paris i s (CaSO4)2 .H2O. Therefore gypsum contains a lower percentage of calcium than plaster of Paris.
Carbonates of alkali and alkaline earth metals differ in:- a)Stability
- b)Lattice enthalpy
- c)Solubility
- d)All the above
Correct answer is option 'D'. Can you explain this answer?
Carbonates of alkali and alkaline earth metals differ in:
a)
Stability
b)
Lattice enthalpy
c)
Solubility
d)
All the above
|
|
Rohan Singh answered |
All the alkali metals have an electron in their outermost shell and all the alkaline earth metals have two outer electrons. To achieve the noble gas configuration, alkali metals need to lose one electron (valence is “one”), whereas alkaline earth metals need to remove two electrons (valence is “two”).
In Castner Kellner cell, electrolyte used is:- a)aq. NaOH
- b)Na/Hg
- c)aq. NaCl
- d)aq. NaBr
Correct answer is option 'C'. Can you explain this answer?
In Castner Kellner cell, electrolyte used is:
a)
aq. NaOH
b)
Na/Hg
c)
aq. NaCl
d)
aq. NaBr
|
|
Riya Banerjee answered |
In castner-kellner method NaOH is prepared by the electrolysis of aqueous solution of NaCl(Brine).
Name the element which forms the stable fluoro complex anions:- a)Ba
- b)Sr
- c)Ca
- d)Be
Correct answer is option 'D'. Can you explain this answer?
Name the element which forms the stable fluoro complex anions:
a)
Ba
b)
Sr
c)
Ca
d)
Be
|
|
Sinjini Shah answered |
The correct answer is option 'D' - Be (Beryllium).
Explanation:
Beryllium (Be) is the element that forms stable fluoro complex anions.
Stable fluoro complex anions are formed when a metal cation (positively charged metal ion) forms a complex with one or more fluoride ions (F-). These complex anions are stable because the metal ion and fluoride ions are held together by strong electrostatic forces.
Beryllium is a unique element in the periodic table because it has a small atomic size and a high charge density. This makes it capable of forming stable complexes with fluoride ions. The small size of the beryllium ion allows for strong interactions with the fluoride ions, resulting in the formation of stable fluoro complex anions.
Other elements like Ba (Barium), Sr (Strontium), and Ca (Calcium) also belong to the alkaline earth metal group and have similar chemical properties to beryllium. However, they have larger atomic sizes and lower charge densities compared to beryllium. As a result, these elements are not able to form stable fluoro complex anions to the same extent as beryllium.
In summary, beryllium is the element that forms stable fluoro complex anions due to its small atomic size and high charge density. Ba, Sr, and Ca, while belonging to the same group, are not capable of forming such stable complexes to the same extent as beryllium.
Explanation:
Beryllium (Be) is the element that forms stable fluoro complex anions.
Stable fluoro complex anions are formed when a metal cation (positively charged metal ion) forms a complex with one or more fluoride ions (F-). These complex anions are stable because the metal ion and fluoride ions are held together by strong electrostatic forces.
Beryllium is a unique element in the periodic table because it has a small atomic size and a high charge density. This makes it capable of forming stable complexes with fluoride ions. The small size of the beryllium ion allows for strong interactions with the fluoride ions, resulting in the formation of stable fluoro complex anions.
Other elements like Ba (Barium), Sr (Strontium), and Ca (Calcium) also belong to the alkaline earth metal group and have similar chemical properties to beryllium. However, they have larger atomic sizes and lower charge densities compared to beryllium. As a result, these elements are not able to form stable fluoro complex anions to the same extent as beryllium.
In summary, beryllium is the element that forms stable fluoro complex anions due to its small atomic size and high charge density. Ba, Sr, and Ca, while belonging to the same group, are not capable of forming such stable complexes to the same extent as beryllium.
The nature of the oxide of radium is:- a)Neutral
- b)Basic
- c)Acidic
- d)Amphoteric
Correct answer is option 'B'. Can you explain this answer?
The nature of the oxide of radium is:
a)
Neutral
b)
Basic
c)
Acidic
d)
Amphoteric
|
|
Rajat Kapoor answered |
Radium oxide (RaO) has not been characterized well past its existence, despite oxides being common compounds for the other alkaline earth metals. Radium hydroxide (Ra(OH)2) is the most readily soluble among the alkaline earth hydroxides and is a stronger base than its barium congener, barium hydroxide.
Which one of the following has minimum value of cation/anion ratio. [1993]- a)NaCl
- b)KCl
- c)MgCl2
- d)CaF2
Correct answer is option 'C'. Can you explain this answer?
Which one of the following has minimum value of cation/anion ratio. [1993]
a)
NaCl
b)
KCl
c)
MgCl2
d)
CaF2

|
Prasenjit Pillai answered |
Atomic size of K+ > Ca2+ > Mg2+ and that of Cl– > F–. Therefore, Mg2+/Cl– ratio has the minimum value.
In Castner Kellner cell:- a)Hg is cathode and carbon is anode
- b)Hg is cathode and anode
- c)Carbon is anode and cathode
- d)Hg is anode and carbon is cathode
Correct answer is option 'A'. Can you explain this answer?
In Castner Kellner cell:
a)
Hg is cathode and carbon is anode
b)
Hg is cathode and anode
c)
Carbon is anode and cathode
d)
Hg is anode and carbon is cathode
|
|
Rahul Bansal answered |
An electrolytic cell used industrially for the production of sodium hydroxide. The usually iron cell is filled with brine (sodium chloride solution) and employs liquid mercury as the cathode. The sodium liberated there forms an amalgam with the mercury, which is run off and reacted with water to give sodium hydroxide (and hydrogen); the mercury is then re-used. Chlorine gas produced at the anode is another valuable by-product.
Mg(NO3)2 on thermal decomposition yields:- a)MgNO2
- b)NO2
- c)N2O
- d)NO
Correct answer is option 'B'. Can you explain this answer?
Mg(NO3)2 on thermal decomposition yields:
a)
MgNO2
b)
NO2
c)
N2O
d)
NO
|
|
Vijay Bansal answered |
2Mg(NO3)2 → 2MgO + 4NO2 + O2
In the replacement reaction The reaction will be most favourable if M happens to be : [2012 M]
The reaction will be most favourable if M happens to be : [2012 M]- a)Na
- b)K
- c)Rb
- d)Li
Correct answer is option 'C'. Can you explain this answer?
In the replacement reaction

The reaction will be most favourable if M happens to be : [2012 M]
a)
Na
b)
K
c)
Rb
d)
Li

|
Tejas Chavan answered |
Tertiary halide can show ionic reaction with MF so, MF should be most ionic for reaction to proceed forward. Hence ‘M’ should be ‘Rb’.
Pure NaCl is obtained by the process of:- a)Sublimation
- b)Crystallization
- c)Magnetic separation
- d)Chromatography
Correct answer is option 'B'. Can you explain this answer?
Pure NaCl is obtained by the process of:
a)
Sublimation
b)
Crystallization
c)
Magnetic separation
d)
Chromatography
|
|
Vijay Bansal answered |
Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering, crystallization occurs in a crystallizer.
Potassium ions are the most abundant cations. They:- a)are the most abundant anions within cell fluids
- b)participate in the oxidation of glucose to produce ATP
- c)inactivate many enzymes which help in penetration of cell membrane
- d)found primarily on the outside of cells
Correct answer is option 'B'. Can you explain this answer?
Potassium ions are the most abundant cations. They:
a)
are the most abundant anions within cell fluids
b)
participate in the oxidation of glucose to produce ATP
c)
inactivate many enzymes which help in penetration of cell membrane
d)
found primarily on the outside of cells
|
|
Preeti Iyer answered |
Potassium ions are the most abundant cations with in cell fluids, where they activate many enzymes and participate in the oxidation of glucose to produce ATP. Potassium ions also participate in the synthesis of proteins.
The lighter alkaline earth metals are very important in animal and plant physiology. Magnesium functions as the active center in some enzymes and calcium salts take a structural role in bones. The alkaline earth metals have the second lowest first ionisation energies in their respective periods. The ions of these elements perform important biological functions like maintenance of ion balance and nerve impulse conduction. The given questions (i) to (iv) consist of an assertion (A) and reason (R). Choose the correct option.Assertion: Be and Mg impart colour to the flame.Reason: They have high ionisation energies.- a)A and R both are correct and R is the correct explanation of A.
- b)A and R both are correct but R is not the correct explanation of A.
- c)A is true but R is false.
- d)A is false but R is true.
Correct answer is option 'A'. Can you explain this answer?
The lighter alkaline earth metals are very important in animal and plant physiology. Magnesium functions as the active center in some enzymes and calcium salts take a structural role in bones. The alkaline earth metals have the second lowest first ionisation energies in their respective periods. The ions of these elements perform important biological functions like maintenance of ion balance and nerve impulse conduction. The given questions (i) to (iv) consist of an assertion (A) and reason (R). Choose the correct option.
Assertion: Be and Mg impart colour to the flame.
Reason: They have high ionisation energies.
a)
A and R both are correct and R is the correct explanation of A.
b)
A and R both are correct but R is not the correct explanation of A.
c)
A is true but R is false.
d)
A is false but R is true.
|
|
Preeti Iyer answered |
Small sized Be and Mg atoms have high ionization energies. Hence, the energy of flame is not sufficient to excite their electrons. Thus, Be and Mg do not produce colour in flame.
Property of the alkaline earth metals that increases with their atomic number : [2010]- a)Solubility of their hydroxides in water
- b)Solubility of their sulphates in water
- c)Ionization energy
- d)Electronegativity
Correct answer is option 'A'. Can you explain this answer?
Property of the alkaline earth metals that increases with their atomic number : [2010]
a)
Solubility of their hydroxides in water
b)
Solubility of their sulphates in water
c)
Ionization energy
d)
Electronegativity

|
Arpita Tiwari answered |
Lattice energy decreases more rapidly than hydration energy for alkaline earth metal hydroxides.
When sodium is dissolved in liquid ammonia, a solution of deep blue colour is obtained. The colour of the solution is due to- a)ammoniated electron
- b)sodium ion
- c)sodium amide
- d)ammoniated sodium ion
Correct answer is option 'A'. Can you explain this answer?
When sodium is dissolved in liquid ammonia, a solution of deep blue colour is obtained. The colour of the solution is due to
a)
ammoniated electron
b)
sodium ion
c)
sodium amide
d)
ammoniated sodium ion

|
Nabanita Basu answered |
Be2+ → Al3+ + Be2+ is diagonaly similar with Al3+ & Al3+ is amphoteric in nature.
A solid compound ‘X’ on heating gives CO2 gas and a residue. The residue mixed with water forms ‘Y’. On passing an excess of CO2 through ‘Y’ in water, a clear solution ‘Z’, is obtained. On boiling ‘Z’, a compound ‘X’ is reformed. The compound ‘X’ is[2004]- a)Ca(HCO3)2
- b)CaCO3
- c)Na2CO3
- d)K2CO3
Correct answer is option 'B'. Can you explain this answer?
A solid compound ‘X’ on heating gives CO2 gas and a residue. The residue mixed with water forms ‘Y’. On passing an excess of CO2 through ‘Y’ in water, a clear solution ‘Z’, is obtained. On boiling ‘Z’, a compound ‘X’ is reformed. The compound ‘X’ is[2004]
a)
Ca(HCO3)2
b)
CaCO3
c)
Na2CO3
d)
K2CO3

|
Arpita Tiwari answered |
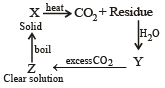
The given properties coincide with CaCO3
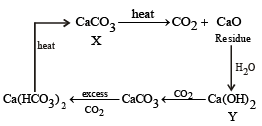
BeCl2 exists as a polymer in:- a)Liquid state
- b)Vapour state
- c)Solid state
- d)All the states
Correct answer is 'C'. Can you explain this answer?
BeCl2 exists as a polymer in:
a)
Liquid state
b)
Vapour state
c)
Solid state
d)
All the states
|
|
Mira Sharma answered |
BeCl2 exists as a polymer in solid state. In this case Be atom is tetrahedrally surrounded by four chlorine atoms.
Which of the following is known as fusion mixture? [1994]- a)Mixture of Na2CO3 + NaHCO3
- b)Na2CO3.10H2O
- c)Mixture of K2CO3 + Na2CO3
- d)NaHCO3
Correct answer is option 'C'. Can you explain this answer?
Which of the following is known as fusion mixture? [1994]
a)
Mixture of Na2CO3 + NaHCO3
b)
Na2CO3.10H2O
c)
Mixture of K2CO3 + Na2CO3
d)
NaHCO3

|
Krish Chakraborty answered |
Mixture of K2CO3 and Na2CO3 is called fusion mixture
The general electronic configuration of the outermost orbit in the case of alkaline earth metal is:- a)ns1
- b)ns2np4
- c)ns2np1
- d)ns2
Correct answer is option 'D'. Can you explain this answer?
The general electronic configuration of the outermost orbit in the case of alkaline earth metal is:
a)
ns1
b)
ns2np4
c)
ns2np1
d)
ns2
|
|
Om Desai answered |
The elements in Group 2 (beryllium, magnesium, calcium, strontium, barium, and radium) are called the alkaline earth metals . These elements have two valence electrons, both of which reside in the outermost s sublevel. The general electron configuration of all alkaline earth metals is ns2.
The alkali metals are low melting. Which of the following alkali metal is expected to melt if the room temperature rises to 30°C?- a)Na
- b)K
- c)Rb
- d)Cs
Correct answer is option 'D'. Can you explain this answer?
The alkali metals are low melting. Which of the following alkali metal is expected to melt if the room temperature rises to 30°C?
a)
Na
b)
K
c)
Rb
d)
Cs
|
|
Anjana Sharma answered |
Among alkali metals, melting point decreases as the strength of metallic bonding decreases with increasing size of the atom. Thus, Cs has the lowest melting point (28.5degreeC) and will melt at 30degreeC.
We can get beryllium hydride by:- a)Treating Be with LiAlH4
- b)Treating BeCl2 with H2
- c)Treating BeCl2 with LiAlH4
- d)Treating Be with H2
Correct answer is option 'C'. Can you explain this answer?
We can get beryllium hydride by:
a)
Treating Be with LiAlH4
b)
Treating BeCl2 with H2
c)
Treating BeCl2 with LiAlH4
d)
Treating Be with H2

|
Milan Datta answered |
To obtain beryllium hydride (BeH2), we can treat beryllium chloride (BeCl2) with lithium aluminum hydride (LiAlH4). Let's break down the process step-by-step:
1. Formation of Lithium Aluminum Hydride (LiAlH4):
- Lithium aluminum hydride is formed by reacting aluminum chloride (AlCl3) with lithium hydride (LiH) in a 1:1 molar ratio.
- The reaction is as follows:
4LiH + AlCl3 → LiAlH4 + 3LiCl
2. Reaction between Beryllium Chloride (BeCl2) and Lithium Aluminum Hydride (LiAlH4):
- Beryllium chloride reacts with lithium aluminum hydride to form beryllium hydride and lithium chloride (LiCl).
- The reaction is as follows:
BeCl2 + LiAlH4 → BeH2 + LiCl + AlCl3
Explanation of the Correct Answer (Option C):
- Option C, which involves treating beryllium chloride with lithium aluminum hydride, is the correct answer.
- This is because the reaction between BeCl2 and LiAlH4 yields beryllium hydride (BeH2), as well as lithium chloride (LiCl) and aluminum chloride (AlCl3) as byproducts.
Summary:
- Beryllium hydride (BeH2) can be obtained by treating beryllium chloride (BeCl2) with lithium aluminum hydride (LiAlH4).
- The reaction between BeCl2 and LiAlH4 results in the formation of BeH2, LiCl, and AlCl3.
Note: Beryllium hydride is a highly reactive compound and should be handled with caution due to its potential health hazards.
1. Formation of Lithium Aluminum Hydride (LiAlH4):
- Lithium aluminum hydride is formed by reacting aluminum chloride (AlCl3) with lithium hydride (LiH) in a 1:1 molar ratio.
- The reaction is as follows:
4LiH + AlCl3 → LiAlH4 + 3LiCl
2. Reaction between Beryllium Chloride (BeCl2) and Lithium Aluminum Hydride (LiAlH4):
- Beryllium chloride reacts with lithium aluminum hydride to form beryllium hydride and lithium chloride (LiCl).
- The reaction is as follows:
BeCl2 + LiAlH4 → BeH2 + LiCl + AlCl3
Explanation of the Correct Answer (Option C):
- Option C, which involves treating beryllium chloride with lithium aluminum hydride, is the correct answer.
- This is because the reaction between BeCl2 and LiAlH4 yields beryllium hydride (BeH2), as well as lithium chloride (LiCl) and aluminum chloride (AlCl3) as byproducts.
Summary:
- Beryllium hydride (BeH2) can be obtained by treating beryllium chloride (BeCl2) with lithium aluminum hydride (LiAlH4).
- The reaction between BeCl2 and LiAlH4 results in the formation of BeH2, LiCl, and AlCl3.
Note: Beryllium hydride is a highly reactive compound and should be handled with caution due to its potential health hazards.
Compared with the alkaline earth metals, the alkali metals exhibit [1990]- a)Smaller ionic radii
- b)Highest boiling points
- c)Greater hardness
- d)Lower ionization energies.
Correct answer is option 'D'. Can you explain this answer?
Compared with the alkaline earth metals, the alkali metals exhibit [1990]
a)
Smaller ionic radii
b)
Highest boiling points
c)
Greater hardness
d)
Lower ionization energies.

|
Dipanjan Chawla answered |
Because of larger size and smaller nuclear charge, alkali metals have low ionization potential relative to alkaline earth metals.
Li and Mg both form chlorides which are:- a)Highly ionic
- b)Unstable
- c)Alkaline
- d)Deliquescent
Correct answer is option 'D'. Can you explain this answer?
Li and Mg both form chlorides which are:
a)
Highly ionic
b)
Unstable
c)
Alkaline
d)
Deliquescent
|
|
Mira Sharma answered |
Chlorides of both Li and Mg are deliquescent (absorb moisture from surroundings) and soluble in alcohol and pyridine. Lithium chloride, like magnesium chloride (MgCl2.6H2O) separates out from hydrated crystal LiCl.2H2O.
Several sodium compounds find use in industries. Which of the following ompounds are used for textile industry? - a)Na2CO3
- b)NaCl
- c)Na2SO4
- d)NaHCO3
Correct answer is option 'A'. Can you explain this answer?
Several sodium compounds find use in industries. Which of the following ompounds are used for textile industry?
a)
Na2CO3
b)
NaCl
c)
Na2SO4
d)
NaHCO3
|
|
Pooja Mehta answered |
Sodium Hydroxide, NaOH
Sodium hydroxide is an important compound of sodium with a wide range of usage.
Production of NaOH by Electrolysis of Brine:
This process is used for the industrial production of NaOH
Chapter doubts & questions for s- block Elements - Inorganic Chemistry for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of s- block Elements - Inorganic Chemistry for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup












