All Exams >
SSS 1 >
Chemistry for SSS 1 >
All Questions
All questions of Acids, Bases and Salts for SSS 1 Exam
Which of the following does not conduct electricity?- a)Sodium hydroxide
- b)Rain water
- c)Hydrochloric acid
- d)Distilled water
Correct answer is option 'D'. Can you explain this answer?
Which of the following does not conduct electricity?
a)
Sodium hydroxide
b)
Rain water
c)
Hydrochloric acid
d)
Distilled water
|
|
Gaurav Kumar answered |
Distilled water do not conduct electricity. The reason is that a liquid conducts electricity is by the positively or negatively charged ions that are actually moving from one of the electrodes to the other, carrying charge (electricity) with them.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following base is used in the manufacture of bleaching powder?- a)Magnesium hydroxide
- b)Sodium hydroxide
- c)Potassium hydroxide
- d)Calcium hydroxide
Correct answer is option 'D'. Can you explain this answer?
Which of the following base is used in the manufacture of bleaching powder?
a)
Magnesium hydroxide
b)
Sodium hydroxide
c)
Potassium hydroxide
d)
Calcium hydroxide

|
Aditya Kumar answered |
Because.... ca(OH)2+ cl2= caocl2 + h2o that's why
Identify ‘X’ in the reaction: 2HCl + CuO → X + H2O- a)CuCl
- b)Cu(OH)2
- c)CuCl2
- d)HOCl
Correct answer is option 'C'. Can you explain this answer?
Identify ‘X’ in the reaction: 2HCl + CuO → X + H2O
a)
CuCl
b)
Cu(OH)2
c)
CuCl2
d)
HOCl
|
|
Vikram Kapoor answered |
When copper oxide and dilute hydrochloric acid are mixed the blue green solution is formed.
The reaction is :-
CuO + 2HCl → CuCl2 + H2O
The reaction is :-
CuO + 2HCl → CuCl2 + H2O
The colour of phenolphthalein in acids is:- a)Colourless
- b)Red
- c)Pink
- d)Blue
Correct answer is option 'A'. Can you explain this answer?
The colour of phenolphthalein in acids is:
a)
Colourless
b)
Red
c)
Pink
d)
Blue
|
|
Krishna Iyer answered |
Phenolphthalein is often used as an indicator in acid–base titrations. For this application, it turns colourless in acidic solutions and magenta in basic solutions.
Carbon dioxide is an example of:- a)Amphoteric oxide
- b)Acidic oxide
- c)Basic oxide
- d)Neutral oxide
Correct answer is option 'B'. Can you explain this answer?
Carbon dioxide is an example of:
a)
Amphoteric oxide
b)
Acidic oxide
c)
Basic oxide
d)
Neutral oxide
|
|
Gaurav Kumar answered |
Acid oxides is a complex chemical substance oxides, which form a salt with the chemical reactions with bases or basic oxides and do not react with acidic oxides.
Examples of acidic oxides can be:
CO2 (all known carbon dioxide), P2O5 - oxide of phosphorus (formed in air if burns white phosphorus), SO3 - oxide of sulfur (VI) is a substance used for sulfuric acid.
Examples of acidic oxides can be:
CO2 (all known carbon dioxide), P2O5 - oxide of phosphorus (formed in air if burns white phosphorus), SO3 - oxide of sulfur (VI) is a substance used for sulfuric acid.
pH of H20 is- a)7
- b)8
- c)9
- d)10
Correct answer is option 'A'. Can you explain this answer?
pH of H20 is
a)
7
b)
8
c)
9
d)
10
|
|
Raghav Bansal answered |
pH of H2O is 7 because it is neutral. It is formed by neutralization reaction.
Identify the type of reaction: HCl + NaOH → NaCl + H2O- a)Combination reaction
- b)Double decomposition reaction
- c)Decomposition reaction
- d)Neutralisation reaction
Correct answer is option 'D'. Can you explain this answer?
Identify the type of reaction: HCl + NaOH → NaCl + H2O
a)
Combination reaction
b)
Double decomposition reaction
c)
Decomposition reaction
d)
Neutralisation reaction
|
|
Krishna Iyer answered |
Reaction of a strong acid with strong base is called neutralization reaction which produces salt and water,
HCl + NaOH → NaCl + H2O
This equation is already balanced.
Aqueous solution of sodium hydroxide turns blue litmus:- a)Red
- b)No change
- c)Colourless
- d)Pink
Correct answer is option 'B'. Can you explain this answer?
Aqueous solution of sodium hydroxide turns blue litmus:
a)
Red
b)
No change
c)
Colourless
d)
Pink

|
Sagar Rane answered |
Since Sodium hydroxide is a base and thus it has no effect on a blue litmus paper but it changes red litmus to blue.
Which of the following is an olfactory indicator?- a)Litmus
- b)Phenolphthalein
- c)Onion
- d)Methyl orange
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an olfactory indicator?
a)
Litmus
b)
Phenolphthalein
c)
Onion
d)
Methyl orange
|
|
Amit Sharma answered |
An olfactory indicator is a material whose smell varies reliant on whether it is mixed with an acidic or basic solution. Olfactory indicators mainly used in laboratory to test whether a solution is a base or an acid. Onion is an example of olfactory indicators.
Which of the following are present in a dilute aqueous solution of hydrochloric acid ?- a)H3O+ + Cl-
- b)H3O+ + CH-
- c)Cl- + OH-
- d)Unionised HCl
Correct answer is option 'A'. Can you explain this answer?
Which of the following are present in a dilute aqueous solution of hydrochloric acid ?
a)
H3O+ + Cl-
b)
H3O+ + CH-
c)
Cl- + OH-
d)
Unionised HCl
|
|
Rohit Sharma answered |
Hydrochloric acid ionises as follows :
HCl→ H+ +Cl-
H+ ions combine with water molecules to form H3O+. Therefore, the ions present in dilute aqueous solution of hydrochloric acid are H3O+ and Cl-.
HCl→ H+ +Cl-
H+ ions combine with water molecules to form H3O+. Therefore, the ions present in dilute aqueous solution of hydrochloric acid are H3O+ and Cl-.
An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change ?- a)Baking powder
- b)Lime
- c)Ammonium hydroxide solution
- d)Hydrochloric acid
Correct answer is option 'D'. Can you explain this answer?
An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change ?
a)
Baking powder
b)
Lime
c)
Ammonium hydroxide solution
d)
Hydrochloric acid
|
|
Pooja Shah answered |
As the solution turns red litmus solution blue, it must be basic. To reverse the change, we must add an acid.
NaOH is obtained by electrolysis of- a)Aq. solution of NaCl
- b)Aq. Na2CO3
- c)Aq. NaHCO3
- d)Molten NaCl
Correct answer is option 'A'. Can you explain this answer?
NaOH is obtained by electrolysis of
a)
Aq. solution of NaCl
b)
Aq. Na2CO3
c)
Aq. NaHCO3
d)
Molten NaCl
|
|
Raghav Bansal answered |
2NaCl + 2H2O → 2NaOH + H2 + Cl2
(Chlor-alkali process)
(Chlor-alkali process)
A piece of granulated zinc was dropped into a copper sulphate solution. After some time, the colour of the solution changed from:- a)light green to blue
- b)blue to colourless
- c)light green to colourless
- d)blue to yellow
Correct answer is option 'B'. Can you explain this answer?
A piece of granulated zinc was dropped into a copper sulphate solution. After some time, the colour of the solution changed from:
a)
light green to blue
b)
blue to colourless
c)
light green to colourless
d)
blue to yellow
|
|
Krishna Iyer answered |
We should know that borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate. We should know that borax is the main component of detergents, cosmetics, and enamel glazes. We also use it as a fire retardant and anti-fungal compound in the manufacture of fiberglass, as a flux in metallurgy. We should know that borax is composed of sodium, borax, oxygen and water.
We should know that borax can be found as anhydrous, pentahydrate or dehydrated salt. We should note that the anhydrous and decahydrate forms are the most common forms of borax. The borax decahydrate chemical formula is Na2B4O7.10H2O
Na2B4O7.10H2O
. So, from this we can say that option A is correct.
We should know that borax can be found as anhydrous, pentahydrate or dehydrated salt. We should note that the anhydrous and decahydrate forms are the most common forms of borax. The borax decahydrate chemical formula is Na2B4O7.10H2O
Na2B4O7.10H2O
. So, from this we can say that option A is correct.

Ag2S reacts with H2S04 to form- a)AgS04
- b)Ag2S04 + H2S
- c)Ag20 + H2S
- d)Ag0H + H2S
Correct answer is option 'B'. Can you explain this answer?
Ag2S reacts with H2S04 to form
a)
AgS04
b)
Ag2S04 + H2S
c)
Ag20 + H2S
d)
Ag0H + H2S
|
|
Anita Menon answered |
Ag2S + H2SO4 → Ag2SO4 + H2S (Double displacement reaction)
Acids turn blue litmus :- a)Blue
- b)Red
- c)colourless
- d)Pink
Correct answer is 'B'. Can you explain this answer?
Acids turn blue litmus :
a)
Blue
b)
Red
c)
colourless
d)
Pink

|
Ameya Rane answered |
Blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic or alkaline conditions
H2SO4 + 2NaOH → Na2SO4 + 2H2O Above equation is a(i) neutralization reaction(ii) double displacement reaction(iii) decomposition reaction(iv) addition reaction- a)(iii) and (iv)
- b)(i) and (ii)
- c)(i) and (iii)
- d)(ii) and (iv)
Correct answer is option 'B'. Can you explain this answer?
H2SO4 + 2NaOH → Na2SO4 + 2H2O Above equation is a
(i) neutralization reaction
(ii) double displacement reaction
(iii) decomposition reaction
(iv) addition reaction
a)
(iii) and (iv)
b)
(i) and (ii)
c)
(i) and (iii)
d)
(ii) and (iv)
|
|
Abhavya Mishra answered |
In neutralization reaction salt and water is formed,as a product and in double displacement reaction two compounds react by exchanging their ions to form a new compound. In the above reaction both condition occurs so 'b' is the correct answer.
In a double displacement reaction?- a)Ions remains stable
- b)Ions get liberated
- c)Ions are exchange
- d)Ions are not created
Correct answer is option 'C'. Can you explain this answer?
In a double displacement reaction?
a)
Ions remains stable
b)
Ions get liberated
c)
Ions are exchange
d)
Ions are not created
|
|
Gaurav Kumar answered |
Sodium aluminate and hydrogen gas are formed when sodium hydroxide reacts with aluminium metal.
2NaOH + 2Al + 2H2O ⇨ 2NaAlO2 + 2H2
2NaOH + 2Al + 2H2O ⇨ 2NaAlO2 + 2H2
Marble chips reacts with a solution to produce a gas which turns lime water milky. So the solution contains:
- a)Na2SO4
- b)H2SO4
- c)K2SO4
- d)none of these
Correct answer is option 'B'. Can you explain this answer?
Marble chips reacts with a solution to produce a gas which turns lime water milky. So the solution contains:
a)
Na2SO4
b)
H2SO4
c)
K2SO4
d)
none of these
|
|
Rajiv Gupta answered |
Marble chips are the substances that have the formula CaCO3
Calcium carbonate reacts with sulphuric acid to form calcium sulphate and carbon dioxide which turns lime water milky.
Calcium carbonate reacts with sulphuric acid to form calcium sulphate and carbon dioxide which turns lime water milky.
CaCO3 + H2SO4 → CaSO4 + CO2 + H2O
Thermal decomposition of a substance is brought about with the help of- a)reactants
- b)water
- c)wind
- d)heat
Correct answer is option 'D'. Can you explain this answer?
Thermal decomposition of a substance is brought about with the help of
a)
reactants
b)
water
c)
wind
d)
heat
|
|
Ananya Das answered |
pH above 7 confirms the compound to be alkaline in nature so it can be either sodium carbonate or sodium hydroxide as HCl and CH3COOH are acidic in nature. Sodium hydroxide is a strong base, so it has the highest pH about 14 while sodium carbonate is a weak base so it can have pH 9.
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue ?- a)Lemon juice
- b)Vinegar
- c)Common salt
- d)An antacid
Correct answer is option 'D'. Can you explain this answer?
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue ?
a)
Lemon juice
b)
Vinegar
c)
Common salt
d)
An antacid

|
Bhargavi Basak answered |
The solution of soil and water is acidic in nature which is shown by the pH paper giving yellow orange colour. This pH can be determined from a pH paper by comparing it with the colour of the supernatant.
Lemon juice is primarily a rich source of vitamin C which is citric acid.
Vinegar is a mixture of acetic acid and water. It is produced by acid acting bacteria. It is a mild acid.
Common salt is sodium chloride which has sodium ions and chloride ions. So, these three cannot change the pH paper to greenish blue as only base can bring this change.
Antacid is a base which is baking soda or sodium bicarbonate or sodium hydrogen carbonate which can change the pH paper from yellow orange to green while others are acidic in nature.
Assertion : Weak acids have low electrical conductivity.Reason : Strong acids and weak acids have equal concentration of hydrogen ions in their solutions.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
- e)Both Assertion and Reason are false.
Correct answer is option 'C'. Can you explain this answer?
Assertion : Weak acids have low electrical conductivity.
Reason : Strong acids and weak acids have equal concentration of hydrogen ions in their solutions.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
e)
Both Assertion and Reason are false.
|
|
Radha Iyer answered |
Assertion (A) is true but reason (R) is false.
Red cabbage indicator turns _______ in basic solutions.- a)Pink
- b)Green
- c)Blue
- d)Red
Correct answer is 'B'. Can you explain this answer?
Red cabbage indicator turns _______ in basic solutions.
a)
Pink
b)
Green
c)
Blue
d)
Red

|
Ruba answered |
This is a natural indicator which are found in the nature of the plants
Which of the following compound can turn blue litmus solution red?- a)CH3CHO
- b)NaOH
- c)CH3OCH3
- d)CH3COOH
Correct answer is 'D'. Can you explain this answer?
Which of the following compound can turn blue litmus solution red?
a)
CH3CHO
b)
NaOH
c)
CH3OCH3
d)
CH3COOH
|
|
Krishna Iyer answered |
Acid convert blue litmus solution to Red. HCHO, CH3CHO are aldehydes. HCOOH, CH3COOH are carboxylic acids. CH3OH and C2H5OH are alcohols. Out of these only carboxyhc acids would turn blue litmus solution red. So HCOOH and CH3COOH would turn blue litmus solution red.
Acids change the colour of methyl orange to:- a)Colourless
- b)Pink/Red
- c)Blue
- d)Purple
Correct answer is option 'B'. Can you explain this answer?
Acids change the colour of methyl orange to:
a)
Colourless
b)
Pink/Red
c)
Blue
d)
Purple
|
|
Vikas Kumar answered |
Methyl orange is a pH indicator frequently used in titration because of its clear and distinct colour variance at different pH values. Methyl orange shows pink colour in acidic medium and yellow colour in basic medium. Because it changes colour at the pH of a mid strength acid, it is usually used in titration for acids. Unlike a universal indicator, methyl orange does not have a full spectrum of colour change, but it has a sharp end point.
One of the constituents of baking powder is sodium hydrogencarbonate, the other constituent is- a)hydrochloric acid
- b)tartaric acid
- c)acetic acid
- d)sulphuric acid
Correct answer is option 'B'. Can you explain this answer?
One of the constituents of baking powder is sodium hydrogencarbonate, the other constituent is
a)
hydrochloric acid
b)
tartaric acid
c)
acetic acid
d)
sulphuric acid
|
|
Ananya Das answered |
Tartaric acid is a leavening agent which is mixed with baking powder to neutralize the bitterness produced by baking powder. It is an important food additive that is being used today. When dry base powder is added to acid forming a solution, it is mixed with cream of tartar which is chemically known as potassium bitartrate.
Assertion : Phenolphthalein gives pink colour in basic solution.Reason : Phenolphthalein is a natural indicator.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
- e)Both Assertion and Reason are false.
Correct answer is option 'C'. Can you explain this answer?
Assertion : Phenolphthalein gives pink colour in basic solution.
Reason : Phenolphthalein is a natural indicator.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
e)
Both Assertion and Reason are false.
|
|
Radha Iyer answered |
Assertion (A) is true but reason (R) is false.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): When common salt is kept open, it absorbs moisture from the air.
Reason (R): Common salt contains little magnesium chloride.
- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
Correct answer is option 'A'. Can you explain this answer?
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): When common salt is kept open, it absorbs moisture from the air.
Reason (R): Common salt contains little magnesium chloride.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
|
|
Rohit Sharma answered |
Magnesium chloride present in common salt is a deliquescent substance i.e. it absorbs moisture from the air when kept in open.
Assertion : The acidity of Mg(OH)2 is two.
Reason : The acidity of a base is equal to the number of hydroxyl ions.
- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
- e)Both Assertion and Reason are false.
Correct answer is option 'A'. Can you explain this answer?
Assertion : The acidity of Mg(OH)2 is two.
Reason : The acidity of a base is equal to the number of hydroxyl ions.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
e)
Both Assertion and Reason are false.
|
|
Vivek Rana answered |
The number of replaceable hydroxyl (OH−) Ions in a base is called the acidity of that base. Magnesium hydroxide has 2 replaceable hydroxyl ions, therefore it's acidity is 2. Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)
Assertion: HCl gas does not change the colour of dry blue litmus paper.
Reason: HCl gas dissolves in the water present in wet litmus paper to from H+ ions.
- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
- e)Both Assertion and Reason are false.
Correct answer is option 'A'. Can you explain this answer?
Assertion: HCl gas does not change the colour of dry blue litmus paper.
Reason: HCl gas dissolves in the water present in wet litmus paper to from H+ ions.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
e)
Both Assertion and Reason are false.
|
|
Anita Menon answered |
Assertion:
"HCl gas does not change the colour of dry blue litmus paper."
- This statement is true. Dry HCl gas cannot release hydrogen ions (H⁺) because it needs to be dissolved in water to form hydronium ions (H3O+H_3O^+H3O+), which cause the acidic effect. Therefore, HCl gas will not change the colour of dry blue litmus paper.
Reason:
"HCl gas dissolves in the water present in wet litmus paper to form H⁺ ions."
- This statement is also true. When HCl gas dissolves in the water present on wet litmus paper, it dissociates to form H⁺ ions, which can then change the colour of blue litmus to red, indicating an acidic environment.
Conclusion:
Both the assertion and reason are true, and the reason correctly explains the assertion.
Thus, the correct answer is:
Option 1: Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Non-metals + acids - a)react slow
- b)no reaction
- c)react moderately
- d)react violently
Correct answer is option 'B'. Can you explain this answer?
Non-metals + acids
a)
react slow
b)
no reaction
c)
react moderately
d)
react violently
|
|
Amit Sharma answered |
Non-metals do not react with acids. Thus, option b is correct.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:Assertion: After white washing the walls, a shiny white finish on walls is obtained after two to three days.Reason: Calcium Oxide reacts with Carbon dioxide to form Calcium Hydrogen Carbonate which gives shiny white finish.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
Correct answer is option 'C'. Can you explain this answer?
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion: After white washing the walls, a shiny white finish on walls is obtained after two to three days.
Reason: Calcium Oxide reacts with Carbon dioxide to form Calcium Hydrogen Carbonate which gives shiny white finish.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
|
|
Ananya Das answered |
Calcium hydroxide is obtained by reaction of calcium oxide and water.
Which gas is released when acids react with metal carbonates?- a)O2
- b)CO2
- c)CO
- d)H2
Correct answer is option 'B'. Can you explain this answer?
Which gas is released when acids react with metal carbonates?
a)
O2
b)
CO2
c)
CO
d)
H2

|
Siddharth answered |
It is the property acid that ..... when acid react with metal carbonates ....acid + metal carbonates -------> salt +carbon dioxide + water .... so option B is correct.......
Assertion : When zinc is added to dilute hydrochloric acid, hydrogen is given off.
Reason : Hydrogen chloride molecules contain hydrochloric acid and hydrogen atoms.
- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Assertion (A) is true but reason (R) is false.
- c)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- d)Assertion (A) is false but reason (R) is true.
- e)Both Assertion and Reason are false.
Correct answer is option 'B'. Can you explain this answer?
Assertion : When zinc is added to dilute hydrochloric acid, hydrogen is given off.
Reason : Hydrogen chloride molecules contain hydrochloric acid and hydrogen atoms.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Assertion (A) is true but reason (R) is false.
c)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
d)
Assertion (A) is false but reason (R) is true.
e)
Both Assertion and Reason are false.
|
|
Radha Iyer answered |
The correct answer is b) Assertion (A) is true but reason (R) is false.
Explanation:
-
Assertion (A): When zinc is added to dilute hydrochloric acid, hydrogen is indeed given off. This is a chemical reaction where zinc displaces hydrogen from hydrochloric acid, producing zinc chloride and hydrogen gas.
-
Reason (R): The statement that "Hydrogen chloride molecules contain hydrochloric acid and hydrogen atoms" is misleading. Hydrogen chloride (HCl) is a compound consisting of hydrogen and chlorine, but it doesn't accurately explain why hydrogen gas is evolved when zinc reacts with dilute HCl.
Therefore, the assertion is true, but the reason does not correctly explain it.
The reaction Pb(NO3)2 + 2NaI → 2NaNO3 + PbI2 ↓ is classified as- a)Combination reaction
- b)Decomposition reaction
- c)Single replacement reaction
- d)Double replacement reaction
Correct answer is option 'D'. Can you explain this answer?
The reaction Pb(NO3)2 + 2NaI → 2NaNO3 + PbI2 ↓ is classified as
a)
Combination reaction
b)
Decomposition reaction
c)
Single replacement reaction
d)
Double replacement reaction
|
|
Ananya Das answered |
Metal oxides react with acid to form salt and water because most of the metal oxides are basic or amphoteric in nature.
Lime water reacts with chlorine to form- a)CaCl2
- b)CaOCl2
- c)Ca(CIO3)2
- d)CaO2Cl2
Correct answer is option 'B'. Can you explain this answer?
Lime water reacts with chlorine to form
a)
CaCl2
b)
CaOCl2
c)
Ca(CIO3)2
d)
CaO2Cl2

|
Shivani Shah answered |
If the lime water reacts with chlorine, it forms CaOCl2, i.e., bleaching powder (chloride of lime).
Assertion (A): When zinc is added to dilute hydrochloric acid, hydrogen is given off.
Reason (R): Hydrogen chloride molecules contain hydrochloric acid and hydrogen atoms
- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
Correct answer is option 'B'. Can you explain this answer?
Assertion (A): When zinc is added to dilute hydrochloric acid, hydrogen is given off.
Reason (R): Hydrogen chloride molecules contain hydrochloric acid and hydrogen atoms
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
|
|
Aditya Shah answered |
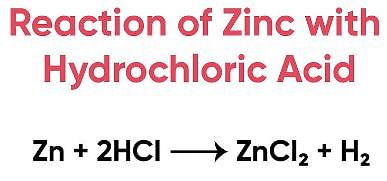
The metal zinc readily reacts with hydrochloric acid to produce hydrogen ga s (H 2 ) and zinc chloride (ZnCl 2 ).
The reaction is given below:
Zn + 2HCl → ZnCl 2 + H 2
Also,
Hydrochloric acid molecules contain chloride and hydroxide ions .
Thus,
- Assertion is true
- Reasoning is true
- And Reasoning is not the correct explanation of Assertion
The reaction, 3MnO2(s) + 4AI(s) → 3Mn(l) + 2Al2O5(s) + Heat is an example of:- a)Combination and exothermic reaction
- b)Combination and endothermic reaction
- c)Displacement and exothermic reaction
- d)Displacement and endothermic reaction.
Correct answer is option 'C'. Can you explain this answer?
The reaction, 3MnO2(s) + 4AI(s) → 3Mn(l) + 2Al2O5(s) + Heat is an example of:
a)
Combination and exothermic reaction
b)
Combination and endothermic reaction
c)
Displacement and exothermic reaction
d)
Displacement and endothermic reaction.
|
|
Pooja Shah answered |
When an acid reacts with a metal, a metal salt and hydrogen are formed. However, this depends if the metal is reactive or not. For example, magnesium, (Mg) is a violently reactive metal to hydrochloric acid) Therefore, making magnesium chloride and hydrogen
Carbon dioxide reacts with calcium hydroxide to form calcium carbonate and water. Ca(OH)2 + CO2 → CaCO3 + H2O This reaction is known as
- a)Neutralization
- b)Decomposition
- c)double decomposition
- d)thermal decomposition
Correct answer is option 'C'. Can you explain this answer?
Carbon dioxide reacts with calcium hydroxide to form calcium carbonate and water. Ca(OH)2 + CO2 → CaCO3 + H2O This reaction is known as
a)
Neutralization
b)
Decomposition
c)
double decomposition
d)
thermal decomposition
|
|
Krishna Iyer answered |
An insoluble milky white precipitate of calcium carbonate and water is produced when calcium hydroxide reacts with carbon dioxide gas. The reaction follows a double displacement reaction.
Assertion : If the pH inside the mouth decreases below5.5, the decay of tooth enamel begins.Reason : The bacteria present in the mouth degrades the sugar and left over food particles and produce acids that remain in the mouth after eating.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
- e)Both Assertion and Reason are false.
Correct answer is option 'A'. Can you explain this answer?
Assertion : If the pH inside the mouth decreases below5.5, the decay of tooth enamel begins.
Reason : The bacteria present in the mouth degrades the sugar and left over food particles and produce acids that remain in the mouth after eating.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
e)
Both Assertion and Reason are false.
|
|
Arun Sharma answered |
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion : Gas bubbles are observed when sodium carbonate is added to dilute hydrochloride acid.Reason : Carbon dioxide is given off in the reaction.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
- e)Both Assertion and Reason are false.
Correct answer is option 'A'. Can you explain this answer?
Assertion : Gas bubbles are observed when sodium carbonate is added to dilute hydrochloride acid.
Reason : Carbon dioxide is given off in the reaction.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
e)
Both Assertion and Reason are false.
|
|
Krishna Iyer answered |
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:Assertion: After white washing the walls, a shiny white finish on walls is obtained after two to three days.Reason: Calcium Oxide reacts with Carbon dioxide to form Calcium Hydrogen Carbonate which gives shiny white finish.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
Correct answer is option 'C'. Can you explain this answer?
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion: After white washing the walls, a shiny white finish on walls is obtained after two to three days.
Reason: Calcium Oxide reacts with Carbon dioxide to form Calcium Hydrogen Carbonate which gives shiny white finish.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
|
|
Rohit Sharma answered |
Calcium hydroxide is obtained by reaction of calcium oxide and water.
Identify the following reactions as endothermic or exothermic,(i) 2NaHCO3 + energy → Na2CO3 + H2O + CO2(ii) 2Mg + O2 → 2MgO + energy- a)(i) Exothermic (ii) Endothermic
- b)(i) Endothermic (ii) Exothermic
- c)(i) Exothermic (ii) Exothermic
- d)(i) Endothermic (ii) Endothermic
Correct answer is option 'B'. Can you explain this answer?
Identify the following reactions as endothermic or exothermic,
(i) 2NaHCO3 + energy → Na2CO3 + H2O + CO2
(ii) 2Mg + O2 → 2MgO + energy
a)
(i) Exothermic (ii) Endothermic
b)
(i) Endothermic (ii) Exothermic
c)
(i) Exothermic (ii) Exothermic
d)
(i) Endothermic (ii) Endothermic
|
|
Krishna Iyer answered |
Ferrous sulphate →FeSO4⋅7H2O
Copper sulphate →CuSO4⋅5H2O
Magnesium sulphate →MgSO4⋅7H2O
Sodium chloride →NaCl
Aluminium can displace Zinc metal from the aqueous solution of zinc sulphate because of ______.- a)zinc is more reactive than aluminium
- b)aluminium is more reactive than zinc
- c)both are equally reactive
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Aluminium can displace Zinc metal from the aqueous solution of zinc sulphate because of ______.
a)
zinc is more reactive than aluminium
b)
aluminium is more reactive than zinc
c)
both are equally reactive
d)
None of the above
|
|
Ananya Das answered |
Seawater contains MgCl2 brine, so salt made from seawater contains this as an impurity, sea salt:
Assertion (A): Baking soda creates acidity in the stomach.Reason (R): Baking soda is alkaline.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
Correct answer is option 'D'. Can you explain this answer?
Assertion (A): Baking soda creates acidity in the stomach.
Reason (R): Baking soda is alkaline.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
|
|
Anita Menon answered |
Baking soda, being alkaline, neutralises the acidity in the stomach and removes it.
Assertion : Plaster of Paris is used by doctors by setting fractured bones.Reason : When Plaster of Paris is mixed with water and applied around the fractured limbs, it sets into a hard mass.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
- e)Both Assertion and Reason are false.
Correct answer is option 'A'. Can you explain this answer?
Assertion : Plaster of Paris is used by doctors by setting fractured bones.
Reason : When Plaster of Paris is mixed with water and applied around the fractured limbs, it sets into a hard mass.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
e)
Both Assertion and Reason are false.
|
|
Krishna Iyer answered |
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A). Plaster of Paris when mixed with water and applied around the fractured limbs, it sets into a hard mass and keeps the bone joints in a fixed position. So, it is commonly used for setting fractured bones.
Assertion (A): Gas bubbles are observed when sodium carbonate is added to dilute hydrochloric acid.Reason (R): Carbon dioxide is given off in the reaction.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
Correct answer is option 'A'. Can you explain this answer?
Assertion (A): Gas bubbles are observed when sodium carbonate is added to dilute hydrochloric acid.
Reason (R): Carbon dioxide is given off in the reaction.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
|
|
Ishan Choudhury answered |
Sodium carbonate reacts with excess hydrochloric acid to form sodium chloride, water and carbon dioxide. In this reaction, bubbles of carbon dioxide are observed.
Which of the following statements are true for acids ?- a)Bitter and change red litmus to blue.
- b)Sour and change red litmus to blue.
- c)Sour and change blue litmus to red.
- d)Bitter and change blue litmus to red.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements are true for acids ?
a)
Bitter and change red litmus to blue.
b)
Sour and change red litmus to blue.
c)
Sour and change blue litmus to red.
d)
Bitter and change blue litmus to red.
|
|
Krishna Iyer answered |
Acids possess sour taste and turn blue litmus paper red.
In a double displacement reaction?- a)Ions remains stable
- b)Ions get liberated
- c)Ions are exchange
- d)Ions are not created
Correct answer is option 'C'. Can you explain this answer?
In a double displacement reaction?
a)
Ions remains stable
b)
Ions get liberated
c)
Ions are exchange
d)
Ions are not created
|
|
Krishna Iyer answered |
When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), salt, water and carbon dioxide are made. In general: Acid + Metal carbonate Salt + Water + Carbon dioxide
Turmeric, a natural indicator in presence of bases turns:
- a)Reddish brown
- b)Blue
- c)No change
- d)Orange
Correct answer is option 'A'. Can you explain this answer?
Turmeric, a natural indicator in presence of bases turns:
a)
Reddish brown
b)
Blue
c)
No change
d)
Orange
|
|
Manoj Choudhury answered |
**Turmeric as a Natural Indicator**
Turmeric, a commonly used spice in cooking, can also be used as a natural indicator to detect the presence of bases. The active compound in turmeric responsible for this property is called curcumin. When curcumin comes into contact with bases, it undergoes a chemical reaction that results in a change in color. In the presence of bases, turmeric turns bright red.
**Explanation**
Turmeric contains a class of compounds known as polyphenols, which are responsible for its vibrant yellow color. Curcumin, one of the polyphenols present in turmeric, acts as a pH indicator. pH indicators are substances that change color depending on the acidity or alkalinity (basicity) of a solution.
Bases are substances that can accept protons or donate hydroxide ions (OH-) in a chemical reaction. When a base is added to a solution of turmeric, the curcumin molecules react with the base, resulting in the formation of a new compound. This new compound has a different structure and absorbs light in a different range of wavelengths, giving rise to a change in color.
In the case of turmeric, the reaction with bases leads to the formation of a red compound. This compound is responsible for the bright red color observed when turmeric comes into contact with bases. The exact mechanism of this reaction is complex and involves multiple steps, including the deprotonation of curcumin and the formation of a conjugated system.
**Conclusion**
In conclusion, turmeric can be used as a natural indicator to detect the presence of bases. When turmeric is exposed to bases, it undergoes a chemical reaction that results in a change in color. The active compound in turmeric, curcumin, reacts with bases to form a red compound, giving turmeric a bright red color in the presence of bases.
Turmeric, a commonly used spice in cooking, can also be used as a natural indicator to detect the presence of bases. The active compound in turmeric responsible for this property is called curcumin. When curcumin comes into contact with bases, it undergoes a chemical reaction that results in a change in color. In the presence of bases, turmeric turns bright red.
**Explanation**
Turmeric contains a class of compounds known as polyphenols, which are responsible for its vibrant yellow color. Curcumin, one of the polyphenols present in turmeric, acts as a pH indicator. pH indicators are substances that change color depending on the acidity or alkalinity (basicity) of a solution.
Bases are substances that can accept protons or donate hydroxide ions (OH-) in a chemical reaction. When a base is added to a solution of turmeric, the curcumin molecules react with the base, resulting in the formation of a new compound. This new compound has a different structure and absorbs light in a different range of wavelengths, giving rise to a change in color.
In the case of turmeric, the reaction with bases leads to the formation of a red compound. This compound is responsible for the bright red color observed when turmeric comes into contact with bases. The exact mechanism of this reaction is complex and involves multiple steps, including the deprotonation of curcumin and the formation of a conjugated system.
**Conclusion**
In conclusion, turmeric can be used as a natural indicator to detect the presence of bases. When turmeric is exposed to bases, it undergoes a chemical reaction that results in a change in color. The active compound in turmeric, curcumin, reacts with bases to form a red compound, giving turmeric a bright red color in the presence of bases.
Chapter doubts & questions for Acids, Bases and Salts - Chemistry for SSS 1 2024 is part of SSS 1 exam preparation. The chapters have been prepared according to the SSS 1 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for SSS 1 2024 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Acids, Bases and Salts - Chemistry for SSS 1 in English & Hindi are available as part of SSS 1 exam.
Download more important topics, notes, lectures and mock test series for SSS 1 Exam by signing up for free.
Chemistry for SSS 1
19 videos|76 docs|22 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup