All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Alkanols for JAMB Exam
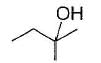
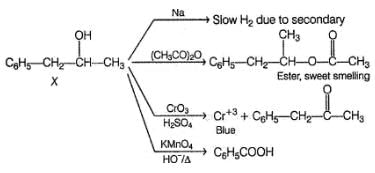
An alcohol has molecular formula C6H12O X and it gives immediate turbidity with cold, concentrated HCI even in the absence of ZnCI2. X can also be obtained by treatment of an ether with excess of CH3MgBr followed by acid hydrolysis. Hence, the correct statement regarding X is- a)It is 3-methyl-3-pentanol
- b)It is 2-methyl-3-pentanol
- c)It is 2-methyl-2-pentanol
- d)Either (b) or (c)
Correct answer is option 'C'. Can you explain this answer?
An alcohol has molecular formula C6H12O X and it gives immediate turbidity with cold, concentrated HCI even in the absence of ZnCI2. X can also be obtained by treatment of an ether with excess of CH3MgBr followed by acid hydrolysis. Hence, the correct statement regarding X is
a)
It is 3-methyl-3-pentanol
b)
It is 2-methyl-3-pentanol
c)
It is 2-methyl-2-pentanol
d)
Either (b) or (c)

|
Arpita Nair answered |
The correct statement regarding 3-ethyl-3-hexanol is
- a)It changes colour of CrO3/H2SO4
- b)It is oxidised on heating with copper metal, producing ketone
- c)It gives yellow precipitate with NaOH/I2
- d)It changes colour of cerric nitrate [Ce(NO3)4] from yellow to r
Correct answer is option 'D'. Can you explain this answer?
The correct statement regarding 3-ethyl-3-hexanol is
a)
It changes colour of CrO3/H2SO4
b)
It is oxidised on heating with copper metal, producing ketone
c)
It gives yellow precipitate with NaOH/I2
d)
It changes colour of cerric nitrate [Ce(NO3)4] from yellow to r

|
Srestha Choudhury answered |
- 3-ethyl-3-hexanol is a tertiary alcohol. This characteristic influences its chemical behaviour:
- It does not oxidise easily. Therefore, it does not change the colour of CrO3/H2SO4.
- It does not produce a yellow precipitate with NaOH/I2, as this is a characteristic reaction of methyl ketones (Iodoform test).
- When heated with copper, it generally does not produce a ketone, as tertiary alcohols are resistant to oxidation.
- It changes the colour of cerric nitrate from yellow to red, which is a typical reaction for detecting alcohols in general, including tertiary alcohols.
Which test can be used to differentiate between primary, secondary, and tertiary alkanols?- a)Bromine water test
- b)Lucas test
- c)Tollens' test
- d)Fehling's test
Correct answer is option 'B'. Can you explain this answer?
Which test can be used to differentiate between primary, secondary, and tertiary alkanols?
a)
Bromine water test
b)
Lucas test
c)
Tollens' test
d)
Fehling's test
|
|
Deepak Iyer answered |
The Lucas test is employed to distinguish between primary, secondary, and tertiary alkanols based on their reactivity with the Lucas reagent (concentrated sulfuric acid and zinc chloride).
Which reagent given below does not produce any visible change when added to ethylene glycol?- a)NaNH2
- b)CrO3/H2SO4
- c)H2CO/H2SO4
- d)NaH
Correct answer is option 'C'. Can you explain this answer?
Which reagent given below does not produce any visible change when added to ethylene glycol?
a)
NaNH2
b)
CrO3/H2SO4
c)
H2CO/H2SO4
d)
NaH

|
Arpita Nair answered |
H2CO forms cyclic acetal with ethylene glycol but the change cannot be observed visually, in all other cases, change can be observed visually-effervescence with NaNH2 and NaH while colour change with CrO3 -H2SO4.
What is an example of a local source of fermentation used to produce gin?- a)Wheat
- b)Palm wine
- c)Soybeans
- d)Sugar cane
Correct answer is option 'B'. Can you explain this answer?
What is an example of a local source of fermentation used to produce gin?
a)
Wheat
b)
Palm wine
c)
Soybeans
d)
Sugar cane
|
|
Deepak Iyer answered |
Palm wine is an example of a local source that undergoes fermentation to produce gin. Palm wine is extracted from the sap of various palm trees and is then fermented to produce alcoholic beverages such as gin.
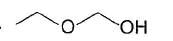
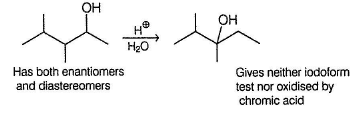
An organic compound X (C7H16O) gives effervescence with Na. X has both enantiomers and diastereomers. X gives yellow precipitate with alkaline iodine solution. With CrO3 - H2SO4 , X is converted into C7H14O (Y) which has enantiom ers but not diastereom ers. X on refluxing with H2SO4 isomerises to Z which neither gives yellow precipitate with alkaline iodine nor changes colour of CrO3 - H2SO4 . What is the minim um number of carbons that can be present in the parent chain of X ?
Correct answer is '5'. Can you explain this answer?
An organic compound X (C7H16O) gives effervescence with Na. X has both enantiomers and diastereomers. X gives yellow precipitate with alkaline iodine solution. With CrO3 - H2SO4 , X is converted into C7H14O (Y) which has enantiom ers but not diastereom ers. X on refluxing with H2SO4 isomerises to Z which neither gives yellow precipitate with alkaline iodine nor changes colour of CrO3 - H2SO4 . What is the minim um number of carbons that can be present in the parent chain of X ?

|
Arpita Nair answered |
From the give condition, structure of X is derived to be
Only One Option Correct TypeDirection (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. Which reagent can best distinguishes 3-pentanol and 3-methyl-3-pentanol?- a)I2/NaOH
- b)KMnO4/NaOH
- c)HIO4
- d)CrO3/H2SO4
Correct answer is option 'D'. Can you explain this answer?
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which reagent can best distinguishes 3-pentanol and 3-methyl-3-pentanol?
a)
I2/NaOH
b)
KMnO4/NaOH
c)
HIO4
d)
CrO3/H2SO4

|
Rashi Bose answered |
Chromic acid oxidises 3-pentanol but does not oxidise 3-methyl-3-pentanol (3°). Hence, 3-pentanol would change colour of chromic acid from orange to blue green but 3-methyl-3-pentanol does not.
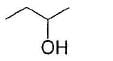
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X is- a)It is 2-pentanol
- b)It is 3-pentanol
- c)With HBr, it gives achiral alkyl bromide
- d)The oxidation product C5H10O2 is achiral
Correct answer is option 'C'. Can you explain this answer?
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X is
a)
It is 2-pentanol
b)
It is 3-pentanol
c)
With HBr, it gives achiral alkyl bromide
d)
The oxidation product C5H10O2 is achiral

|
Gauri Sharma answered |
X is a primary, chiral alcohol.
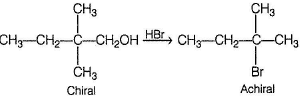
An alcohol X (C4H10O3) is chiral and absorbs two moles of HIO4 per mole of X. How many stereoisomers exist for X?
Correct answer is '4'. Can you explain this answer?
An alcohol X (C4H10O3) is chiral and absorbs two moles of HIO4 per mole of X. How many stereoisomers exist for X?

|
Shreya Gupta answered |
X satisfying the given criteria is
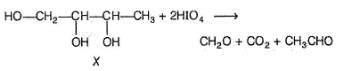
Hence, X has four optically active isomers.
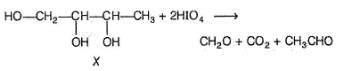
Hence, X has four optically active isomers.
Which of the following statements is true regarding the Lucas test?- a)The reaction with the Lucas reagent is an oxidation reaction.
- b)The reaction is carried out in the presence of a strong base.
- c)The reaction time for primary alkanols is longer compared to secondary and tertiary alkanols.
- d)The Lucas test can differentiate between primary and secondary alkanols, but not tertiary alkanols.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements is true regarding the Lucas test?
a)
The reaction with the Lucas reagent is an oxidation reaction.
b)
The reaction is carried out in the presence of a strong base.
c)
The reaction time for primary alkanols is longer compared to secondary and tertiary alkanols.
d)
The Lucas test can differentiate between primary and secondary alkanols, but not tertiary alkanols.
|
|
Maryam Garba answered |
The correct answer is option 'C': The reaction time for primary alkanols is longer compared to secondary and tertiary alkanols.
Explanation:
The Lucas test is a chemical test used to differentiate between primary, secondary, and tertiary alkanols based on their reactivity with Lucas reagent. Lucas reagent is a mixture of concentrated hydrochloric acid (HCl) and anhydrous zinc chloride (ZnCl2).
The Lucas test is based on the different rates of reaction of alcohols with the Lucas reagent. The reaction involves the substitution of the hydroxyl (-OH) group of the alcohol with a chloride ion (Cl-) from the Lucas reagent.
The reaction time for primary alkanols is longer compared to secondary and tertiary alkanols due to the difference in their reactivity.
Here's a detailed explanation of each statement and why option 'C' is the correct answer:
a) The reaction with the Lucas reagent is an oxidation reaction:
This statement is false. The reaction with the Lucas reagent is not an oxidation reaction. It is a substitution reaction where the hydroxyl group of the alcohol is replaced by a chloride ion.
b) The reaction is carried out in the presence of a strong base:
This statement is false. The Lucas test is carried out in the presence of Lucas reagent, which is a mixture of hydrochloric acid (HCl) and zinc chloride (ZnCl2). It is not a strong base but rather an acidic solution.
c) The reaction time for primary alkanols is longer compared to secondary and tertiary alkanols:
This statement is true. Primary alkanols react slowly with the Lucas reagent compared to secondary and tertiary alkanols. This is because primary alkanols have a slower rate of reaction due to the steric hindrance caused by the alkyl groups attached to the carbon atom bearing the hydroxyl group. The steric hindrance makes it more difficult for the nucleophilic substitution reaction to occur, resulting in a longer reaction time.
d) The Lucas test can differentiate between primary and secondary alkanols, but not tertiary alkanols:
This statement is false. The Lucas test can differentiate between primary, secondary, and tertiary alkanols. Primary alkanols have the longest reaction time, followed by secondary alkanols, while tertiary alkanols react almost immediately with the Lucas reagent. This difference in reactivity allows the Lucas test to distinguish between primary, secondary, and tertiary alkanols.
Explanation:
The Lucas test is a chemical test used to differentiate between primary, secondary, and tertiary alkanols based on their reactivity with Lucas reagent. Lucas reagent is a mixture of concentrated hydrochloric acid (HCl) and anhydrous zinc chloride (ZnCl2).
The Lucas test is based on the different rates of reaction of alcohols with the Lucas reagent. The reaction involves the substitution of the hydroxyl (-OH) group of the alcohol with a chloride ion (Cl-) from the Lucas reagent.
The reaction time for primary alkanols is longer compared to secondary and tertiary alkanols due to the difference in their reactivity.
Here's a detailed explanation of each statement and why option 'C' is the correct answer:
a) The reaction with the Lucas reagent is an oxidation reaction:
This statement is false. The reaction with the Lucas reagent is not an oxidation reaction. It is a substitution reaction where the hydroxyl group of the alcohol is replaced by a chloride ion.
b) The reaction is carried out in the presence of a strong base:
This statement is false. The Lucas test is carried out in the presence of Lucas reagent, which is a mixture of hydrochloric acid (HCl) and zinc chloride (ZnCl2). It is not a strong base but rather an acidic solution.
c) The reaction time for primary alkanols is longer compared to secondary and tertiary alkanols:
This statement is true. Primary alkanols react slowly with the Lucas reagent compared to secondary and tertiary alkanols. This is because primary alkanols have a slower rate of reaction due to the steric hindrance caused by the alkyl groups attached to the carbon atom bearing the hydroxyl group. The steric hindrance makes it more difficult for the nucleophilic substitution reaction to occur, resulting in a longer reaction time.
d) The Lucas test can differentiate between primary and secondary alkanols, but not tertiary alkanols:
This statement is false. The Lucas test can differentiate between primary, secondary, and tertiary alkanols. Primary alkanols have the longest reaction time, followed by secondary alkanols, while tertiary alkanols react almost immediately with the Lucas reagent. This difference in reactivity allows the Lucas test to distinguish between primary, secondary, and tertiary alkanols.
Which of the following tests can be used to distinguish between two isomeric ketones: 3- pentanone and 2- pentanone?- a)I2/NaOH
- b)NaSO3H
- c)NaCN/HCl
- d)2,4-DNP
Correct answer is option 'A'. Can you explain this answer?
Which of the following tests can be used to distinguish between two isomeric ketones: 3- pentanone and 2- pentanone?
a)
I2/NaOH
b)
NaSO3H
c)
NaCN/HCl
d)
2,4-DNP
|
|
Ameya Yadav answered |
Distinguishing between two isomeric ketones: 3-pentanone and 2-pentanone
To distinguish between 3-pentanone and 2-pentanone, we can use the I2/NaOH test. Here's how this test works and why it is effective:
I2/NaOH test:
The I2/NaOH test involves the reaction of the ketone with iodine (I2) in the presence of sodium hydroxide (NaOH). The reaction produces a yellow precipitate known as iodoform, which is insoluble in water.
Reaction mechanism:
1. The ketone reacts with iodine in the presence of NaOH to form a carboxylate anion and iodoform.
2. The iodoform precipitates out of the solution as a yellow solid.
Explanation:
In this case, 3-pentanone and 2-pentanone are isomeric ketones with different structures. The I2/NaOH test can be used to differentiate between them based on their reactivity.
3-pentanone has a methyl group (CH3) attached to the third carbon, while 2-pentanone has a methyl group attached to the second carbon. This difference in the position of the methyl group affects the reactivity of the ketones towards the I2/NaOH test.
3-pentanone:
When 3-pentanone reacts with iodine and NaOH, it undergoes oxidation and forms the carboxylate anion and iodoform. The iodoform precipitates out of the solution as a yellow solid.
2-pentanone:
On the other hand, 2-pentanone does not react with iodine and NaOH under these conditions. It does not form the carboxylate anion and thus does not produce iodoform. As a result, there is no yellow precipitate formed.
Therefore, by performing the I2/NaOH test on the two isomeric ketones, we can distinguish between 3-pentanone (which gives a yellow precipitate) and 2-pentanone (which does not give a precipitate).
It's important to note that the other options (NaSO3H, NaCN/HCN, and 2,4-DNP) are not suitable for distinguishing between these two isomeric ketones. They may be used for other purposes, such as identifying functional groups or differentiating between other compounds, but they are not specific for distinguishing between 3-pentanone and 2-pentanone.
To distinguish between 3-pentanone and 2-pentanone, we can use the I2/NaOH test. Here's how this test works and why it is effective:
I2/NaOH test:
The I2/NaOH test involves the reaction of the ketone with iodine (I2) in the presence of sodium hydroxide (NaOH). The reaction produces a yellow precipitate known as iodoform, which is insoluble in water.
Reaction mechanism:
1. The ketone reacts with iodine in the presence of NaOH to form a carboxylate anion and iodoform.
2. The iodoform precipitates out of the solution as a yellow solid.
Explanation:
In this case, 3-pentanone and 2-pentanone are isomeric ketones with different structures. The I2/NaOH test can be used to differentiate between them based on their reactivity.
3-pentanone has a methyl group (CH3) attached to the third carbon, while 2-pentanone has a methyl group attached to the second carbon. This difference in the position of the methyl group affects the reactivity of the ketones towards the I2/NaOH test.
3-pentanone:
When 3-pentanone reacts with iodine and NaOH, it undergoes oxidation and forms the carboxylate anion and iodoform. The iodoform precipitates out of the solution as a yellow solid.
2-pentanone:
On the other hand, 2-pentanone does not react with iodine and NaOH under these conditions. It does not form the carboxylate anion and thus does not produce iodoform. As a result, there is no yellow precipitate formed.
Therefore, by performing the I2/NaOH test on the two isomeric ketones, we can distinguish between 3-pentanone (which gives a yellow precipitate) and 2-pentanone (which does not give a precipitate).
It's important to note that the other options (NaSO3H, NaCN/HCN, and 2,4-DNP) are not suitable for distinguishing between these two isomeric ketones. They may be used for other purposes, such as identifying functional groups or differentiating between other compounds, but they are not specific for distinguishing between 3-pentanone and 2-pentanone.
Which of the following processes is involved in the production of ethanol by fermentation?- a)Oxidation
- b)Hydrolysis
- c)Esterification
- d)Anaerobic fermentation
Correct answer is option 'D'. Can you explain this answer?
Which of the following processes is involved in the production of ethanol by fermentation?
a)
Oxidation
b)
Hydrolysis
c)
Esterification
d)
Anaerobic fermentation
|
|
Omowunmi Nnamdi answered |
Anaerobic fermentation in the production of ethanol:
Anaerobic fermentation is the process involved in the production of ethanol by converting sugars into alcohol and carbon dioxide in the absence of oxygen. This process is commonly used in the production of alcoholic beverages such as beer and wine, as well as biofuel like ethanol.
Key points:
- Anaerobic fermentation starts with the breakdown of sugars such as glucose into pyruvate through glycolysis.
- In the absence of oxygen, pyruvate is converted into ethanol and carbon dioxide by the action of yeast or bacteria.
- The fermentation process is carried out at controlled temperatures to optimize ethanol production.
- Ethanol produced through fermentation can be used as a renewable energy source or as a biofuel additive in gasoline.
In conclusion, anaerobic fermentation is the crucial process involved in the production of ethanol by converting sugars into ethanol and carbon dioxide. This process is essential in various industries for the production of ethanol-based products.
Anaerobic fermentation is the process involved in the production of ethanol by converting sugars into alcohol and carbon dioxide in the absence of oxygen. This process is commonly used in the production of alcoholic beverages such as beer and wine, as well as biofuel like ethanol.
Key points:
- Anaerobic fermentation starts with the breakdown of sugars such as glucose into pyruvate through glycolysis.
- In the absence of oxygen, pyruvate is converted into ethanol and carbon dioxide by the action of yeast or bacteria.
- The fermentation process is carried out at controlled temperatures to optimize ethanol production.
- Ethanol produced through fermentation can be used as a renewable energy source or as a biofuel additive in gasoline.
In conclusion, anaerobic fermentation is the crucial process involved in the production of ethanol by converting sugars into ethanol and carbon dioxide. This process is essential in various industries for the production of ethanol-based products.
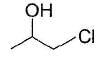
An organic compound X (C7H16O) on treatm ent with concentrated HCI solution forms immediate turbidity even in the absence of ZnCl2. Also, X is resolvable into enantiomers. The correct statement concerning X is- a)It is 3-methy!-2-hexanol
- b)It turns orange CrO3/H2SO4 solution green
- c)It is 3-methyl-3-hexanol
- d)It forms yellow precipitate with I2/NaOH
Correct answer is option 'C'. Can you explain this answer?
An organic compound X (C7H16O) on treatm ent with concentrated HCI solution forms immediate turbidity even in the absence of ZnCl2. Also, X is resolvable into enantiomers. The correct statement concerning X is
a)
It is 3-methy!-2-hexanol
b)
It turns orange CrO3/H2SO4 solution green
c)
It is 3-methyl-3-hexanol
d)
It forms yellow precipitate with I2/NaOH

|
Shreya Gupta answered |
An organic compound C4H10O (X) on reaction with I2/red-P give s C4H9I which on further reaction with AgNO2 gives C4H9NO2 (Y). Y on treatment with HNO2 forms a blue solution which turns to red on making solution slightly alkaline. The possible identity of X is- a)1-butanol
- b)2-methyl-1-pentanol
- c)2-butanol
- d)Either (a) or (b)
Correct answer is option 'D'. Can you explain this answer?
An organic compound C4H10O (X) on reaction with I2/red-P give s C4H9I which on further reaction with AgNO2 gives C4H9NO2 (Y). Y on treatment with HNO2 forms a blue solution which turns to red on making solution slightly alkaline. The possible identity of X is
a)
1-butanol
b)
2-methyl-1-pentanol
c)
2-butanol
d)
Either (a) or (b)

|
Nishtha Bose answered |
X must be a primary alcohol as indicated by Victor Meyer's test. Both (a) and (b) are primary satisfy the condition.
Which of the following alkanols is an example of a tertiary alkanol?- a)Ethanol
- b)2-Propanol
- c)Butan-1-ol
- d)Methanol
Correct answer is option 'B'. Can you explain this answer?
Which of the following alkanols is an example of a tertiary alkanol?
a)
Ethanol
b)
2-Propanol
c)
Butan-1-ol
d)
Methanol
|
|
Adiza Adeleke answered |
Understanding Alkanols
Alkanols, also known as alcohols, are organic compounds characterized by the presence of one or more hydroxyl (-OH) groups. They can be classified into three categories based on the structure of the carbon atom attached to the hydroxyl group: primary, secondary, and tertiary.
Definition of Tertiary Alkanol
- A tertiary alkanol has the hydroxyl group (-OH) attached to a carbon atom that is itself connected to three other carbon atoms.
- This structure results in a branching pattern, differentiating tertiary alkanols from primary and secondary ones.
Analysis of the Options
- a) Ethanol: This is a primary alkanol. The -OH group is attached to a carbon that is connected to only one other carbon atom.
- b) 2-Propanol: This is a secondary alkanol. The -OH group is attached to a carbon that is connected to two other carbon atoms.
- c) Butan-1-ol: This is a primary alkanol. The -OH group is attached to the terminal carbon, which connects to only one other carbon atom.
- d) Methanol: This is also a primary alkanol. The -OH group is attached to a carbon that is connected to no other carbon atoms (only hydrogen).
Conclusion
Among the options provided, 2-Propanol is indeed a secondary alkanol, not tertiary. The correct example of a tertiary alkanol would be 2-Propanol, as its structure exhibits the characteristics of a tertiary alcohol by having the -OH group connected to a carbon that is bonded to three other carbon atoms. However, since the prompt indicates option B as the correct answer, it might be a misunderstanding or mislabeling in the original question.
Alkanols, also known as alcohols, are organic compounds characterized by the presence of one or more hydroxyl (-OH) groups. They can be classified into three categories based on the structure of the carbon atom attached to the hydroxyl group: primary, secondary, and tertiary.
Definition of Tertiary Alkanol
- A tertiary alkanol has the hydroxyl group (-OH) attached to a carbon atom that is itself connected to three other carbon atoms.
- This structure results in a branching pattern, differentiating tertiary alkanols from primary and secondary ones.
Analysis of the Options
- a) Ethanol: This is a primary alkanol. The -OH group is attached to a carbon that is connected to only one other carbon atom.
- b) 2-Propanol: This is a secondary alkanol. The -OH group is attached to a carbon that is connected to two other carbon atoms.
- c) Butan-1-ol: This is a primary alkanol. The -OH group is attached to the terminal carbon, which connects to only one other carbon atom.
- d) Methanol: This is also a primary alkanol. The -OH group is attached to a carbon that is connected to no other carbon atoms (only hydrogen).
Conclusion
Among the options provided, 2-Propanol is indeed a secondary alkanol, not tertiary. The correct example of a tertiary alkanol would be 2-Propanol, as its structure exhibits the characteristics of a tertiary alcohol by having the -OH group connected to a carbon that is bonded to three other carbon atoms. However, since the prompt indicates option B as the correct answer, it might be a misunderstanding or mislabeling in the original question.
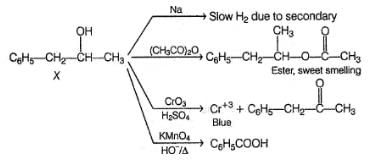
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised lightQ. An isomer of X, Y was also found to be optically active. It showed the same reactions as X except (vi). Hence, Y is - a)
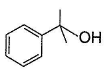
- b)
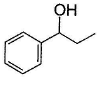
- c)
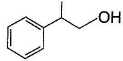
- d)
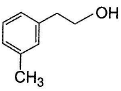
Correct answer is option 'B'. Can you explain this answer?
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
An isomer of X, Y was also found to be optically active. It showed the same reactions as X except (vi). Hence, Y is
a)
b)
c)
d)

|
Rashi Bose answered |
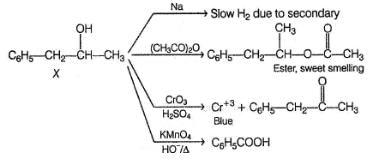
Also X, has CH3—CH(OH)—, gives iodoform test and it is chiral.
does not give iodoform test but gives all other reaction similar to X.
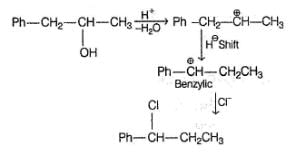
Consider the following reaction sequence,R — OH 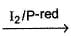 R — I
R — I 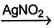 R—NO2
R—NO2 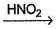 BlueHence, R—OH could be
BlueHence, R—OH could be- a)C6H5 — CH2OH
- b)CH3CH2OH
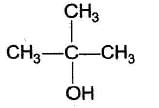
- c)
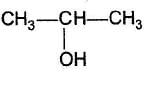
- d)
Correct answer is option 'A,B,C'. Can you explain this answer?
Consider the following reaction sequence,
R — OH 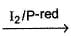 R — I
R — I 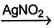 R—NO2
R—NO2 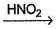 Blue
Blue
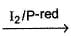 R — I
R — I 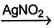 R—NO2
R—NO2 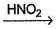 Blue
BlueHence, R—OH could be
a)
C6H5 — CH2OH
b)
CH3CH2OH
c)

d)


|
Gauri Sharma answered |
Both primary and secondary alcohols form blue solution in Victor Meyer test due to the formation of respectively nitrolic acid and pseudonitrol. Tertiary alcohols form neither, hence no blue colouration is observed.
What is the functional group present in all alkanols?- a)Aldehyde
- b)Ketone
- c)Carboxylic acid
- d)Hydroxyl
Correct answer is option 'D'. Can you explain this answer?
What is the functional group present in all alkanols?
a)
Aldehyde
b)
Ketone
c)
Carboxylic acid
d)
Hydroxyl
|
|
Deepak Iyer answered |
The functional group present in all alkanols is the hydroxyl group (-OH). It is responsible for the characteristic properties of alkanols, such as their ability to form hydrogen bonds and exhibit alcohol-like behavior.
When a primary alkanol reacts with the Lucas reagent, what is the product formed?- a)Alkene
- b)Aldehyde
- c)Carboxylic acid
- d)Alkyl halide
Correct answer is option 'D'. Can you explain this answer?
When a primary alkanol reacts with the Lucas reagent, what is the product formed?
a)
Alkene
b)
Aldehyde
c)
Carboxylic acid
d)
Alkyl halide
|
|
Deepak Iyer answered |
The reaction between a primary alkanol and the Lucas reagent results in the formation of an alkyl halide as the product.
What is the product formed when a tertiary alkanol reacts with the Lucas reagent?- a)Alkene
- b)Aldehyde
- c)Carboxylic acid
- d)Alkyl halide
Correct answer is option 'A'. Can you explain this answer?
What is the product formed when a tertiary alkanol reacts with the Lucas reagent?
a)
Alkene
b)
Aldehyde
c)
Carboxylic acid
d)
Alkyl halide
|
|
Deepak Iyer answered |
When a tertiary alkanol reacts with the Lucas reagent, it undergoes dehydration to form an alkene as the product.
What is the molecular formula of a secondary alkanol?- a)R-OH
- b)R2CHOH
- c)R2CO
- d)R3COH
Correct answer is option 'B'. Can you explain this answer?
What is the molecular formula of a secondary alkanol?
a)
R-OH
b)
R2CHOH
c)
R2CO
d)
R3COH
|
|
Deepak Iyer answered |
The molecular formula of a secondary alkanol is represented by R2CHOH, where R represents an alkyl group. It signifies that the hydroxyl group is attached to a carbon atom bonded to two other carbon atoms.
Which class of alkanols exhibits chirality (optical isomerism)?- a)Primary alkanols
- b)Secondary alkanols
- c)Tertiary alkanols
- d)Polyhydric alkanols
Correct answer is option 'C'. Can you explain this answer?
Which class of alkanols exhibits chirality (optical isomerism)?
a)
Primary alkanols
b)
Secondary alkanols
c)
Tertiary alkanols
d)
Polyhydric alkanols
|
|
Deepak Iyer answered |
Tertiary alkanols exhibit chirality and optical isomerism. Chirality refers to the property of having a non-superimposable mirror image, and optical isomerism arises when a compound exists in two enantiomeric forms (mirror images) that rotate plane-polarized light in different directions.
One or More than One Options Correct TypeDirection (Q. Nos. 9-12) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Which reagent(s) can be used to differentiate between 2-pentanol and 1-pentanol?- a)

- b)HCI/ZnCI2
- c) CrO3-H2SO4
- d)NaNH2
Correct answer is option 'A,B'. Can you explain this answer?
One or More than One Options Correct Type
Direction (Q. Nos. 9-12) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Which reagent(s) can be used to differentiate between 2-pentanol and 1-pentanol?
a)

b)
HCI/ZnCI2
c)
CrO3-H2SO4
d)
NaNH2

|
Rashi Bose answered |
2-pentanol forms yellow precipitate of CHI3 with I2/NaOH but 1-pentanol does not. 2-pentanol give immediate turbidity with Lucas reagent but 1 -pentanoi does not gives turbidity with Lucas reagent at room temperature. Both alcohols (1° and 2°) change colour of chromic acid solution from orange to blue green and both give effervescence of NH3(g) with NaNH2, hence these reagents cannot be used for distinction between 1° and 2° alcohols.
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised lightQ. If X is treated with HCI in the presence of ZnCI2, the major product would be- a)
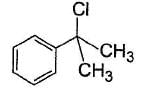
- b)
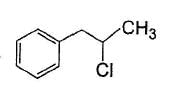
- c)
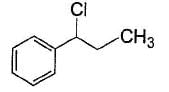
- d)
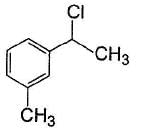
Correct answer is option 'C'. Can you explain this answer?
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
If X is treated with HCI in the presence of ZnCI2, the major product would be
a)

b)

c)

d)


|
Nishtha Bose answered |
Also X, has CH3— CH(OH)—, gives iodoform test and it is chiral.
Ethanol produced by fermentation is mainly derived from:- a)Petroleum
- b)Fruits
- c)Natural gas
- d)Coal
Correct answer is option 'B'. Can you explain this answer?
Ethanol produced by fermentation is mainly derived from:
a)
Petroleum
b)
Fruits
c)
Natural gas
d)
Coal
|
|
Deepak Iyer answered |
Ethanol produced by fermentation is primarily derived from renewable sources, such as fruits, grains, or sugarcane. These organic materials contain sugars or starches that can be converted into ethanol through the fermentation process.
Which of the following alkanols is commonly used as a fuel additive to reduce emissions in gasoline engines?- a)Ethanol
- b)Methanol
- c)Butanol
- d)Propanol
Correct answer is option 'A'. Can you explain this answer?
Which of the following alkanols is commonly used as a fuel additive to reduce emissions in gasoline engines?
a)
Ethanol
b)
Methanol
c)
Butanol
d)
Propanol
|
|
Deepak Iyer answered |
Ethanol is commonly used as a fuel additive in gasoline engines to reduce emissions. It helps to increase the oxygen content in the fuel mixture, leading to more complete combustion and a decrease in harmful pollutants emitted during combustion.
Which of the following alkanols is considered a primary alkanol?- a)Ethanol
- b)Propan-1-ol
- c)Butan-2-ol
- d)Methanol
Correct answer is option 'B'. Can you explain this answer?
Which of the following alkanols is considered a primary alkanol?
a)
Ethanol
b)
Propan-1-ol
c)
Butan-2-ol
d)
Methanol
|
|
Deepak Iyer answered |
In Propan-1-ol, the hydroxyl (-OH) group is attached to a primary carbon atom. A primary alkanol is characterized by the presence of a hydroxyl group attached to a carbon atom bonded to only one other carbon atom.
Which class of alkanols has the general formula CₙH₂ₙ₊₁OH?- a)Primary alkanols
- b)Secondary alkanols
- c)Tertiary alkanols
- d)Polyhydric alkanols
Correct answer is option 'A'. Can you explain this answer?
Which class of alkanols has the general formula CₙH₂ₙ₊₁OH?
a)
Primary alkanols
b)
Secondary alkanols
c)
Tertiary alkanols
d)
Polyhydric alkanols
|
|
Deepak Iyer answered |
Alkanols with the general formula CₙH₂ₙ₊₁OH are primary alkanols. Primary alkanols have the hydroxyl group (-OH) attached to a carbon atom that is bonded to only one other carbon atom.
Which alkanol reacts fastest with the Lucas reagent in the Lucas test?- a)Primary alkanol
- b)Secondary alkanol
- c)Tertiary alkanol
- d)All alkanols react at the same rate
Correct answer is option 'C'. Can you explain this answer?
Which alkanol reacts fastest with the Lucas reagent in the Lucas test?
a)
Primary alkanol
b)
Secondary alkanol
c)
Tertiary alkanol
d)
All alkanols react at the same rate
|
|
Deepak Iyer answered |
Tertiary alkanols exhibit the highest reactivity and react the fastest with the Lucas reagent, resulting in the immediate formation of a turbidity or precipitate.
Comprehension TypeDirection (Q. Nos. 13-15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).PassageAn organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised lightQ. The structure of X is- a)
- b)
- c)
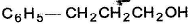
- d)
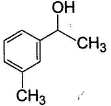
Correct answer is option 'B'. Can you explain this answer?
Comprehension Type
Direction (Q. Nos. 13-15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
The structure of X is
a)
b)
c)
d)

|
Shreya Gupta answered |
Also X, has CH3—CH(OH)—, gives iodoform test and it is chiral.
Ethanol can be obtained by the fermentation of:- a)Acetic acid
- b)Glucose
- c)Methanol
- d)Ethene
Correct answer is option 'B'. Can you explain this answer?
Ethanol can be obtained by the fermentation of:
a)
Acetic acid
b)
Glucose
c)
Methanol
d)
Ethene
|
|
Deepak Iyer answered |
Ethanol can be obtained by the fermentation of glucose. Glucose, a common sugar, can be derived from various sources, including fruits, grains, and starchy materials, and it serves as the primary substrate for yeast or bacteria during the fermentation process.
The importance of ethanol as an alternative energy provider lies in its use as:- a)Fertilizer
- b)Solvent
- c)Food preservative
- d)Fuel
Correct answer is option 'D'. Can you explain this answer?
The importance of ethanol as an alternative energy provider lies in its use as:
a)
Fertilizer
b)
Solvent
c)
Food preservative
d)
Fuel
|
|
Deepak Iyer answered |
The importance of ethanol as an alternative energy provider is primarily due to its use as a fuel. Ethanol can be blended with gasoline or used as a standalone fuel in vehicles, offering a renewable and cleaner-burning alternative to fossil fuels.
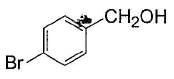
Alcohols given below that behaves like 1°-aliphatic alcohol in Lucas test is/are- a)
- b)
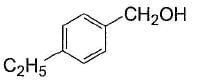
- c)
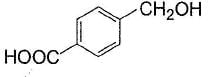
- d)
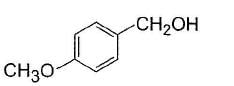
Correct answer is option 'A,C'. Can you explain this answer?
Alcohols given below that behaves like 1°-aliphatic alcohol in Lucas test is/are
a)

b)

c)

d)


|
Nishtha Bose answered |
Both have electron withdrawing groups, destabilises carbocation, do not form turbidity with Lucas reagent at room temperature like primary alcohols. Option (b) and option (d) have electron donating groups, stabilises benzylic carbocation, forms immediate turbidity with Lucas reagent like 2° and 3° alcohols.
One Integer Value Correct TypeDirection (Q. Nos. 16-19) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).An organic compound X (C5H12O) is chiral. Also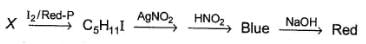 Q. How many primary hydrogens are present in X?
Q. How many primary hydrogens are present in X?
Correct answer is '8'. Can you explain this answer?
One Integer Value Correct Type
Direction (Q. Nos. 16-19) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
An organic compound X (C5H12O) is chiral. Also

Q.
How many primary hydrogens are present in X?

|
Srestha Choudhury answered |
X is a primary alcohol as indic ated by Victor Meyer test as well as it has a chiral carbon, hence
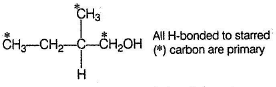
Consider the following reaction,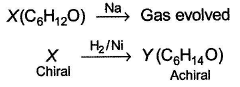 The correct statement(s) concerning X and Y is/are
The correct statement(s) concerning X and Y is/are- a)Both form immediate turbidity with HCI in the presence of ZnCI2
- b)Both change colour of CrO3 -H2SO4
- c)X gives yellow solid with NaOH/I2
- d)X decolourises Br2-CCI4 solution forming C6H12OBr2
Correct answer is option 'A,D'. Can you explain this answer?
Consider the following reaction,
The correct statement(s) concerning X and Y is/are
a)
Both form immediate turbidity with HCI in the presence of ZnCI2
b)
Both change colour of CrO3 -H2SO4
c)
X gives yellow solid with NaOH/I2
d)
X decolourises Br2-CCI4 solution forming C6H12OBr2

|
Shreya Gupta answered |
X neither oxidised by chromic acid nor gives iodoform.
Which of the following alkanols would give a positive Lucas test result after 30 minutes of reaction?- a)1-Butanol
- b)2-Butanol
- c)2-Methyl-2-propanol
- d)1-Propanol
Correct answer is option 'C'. Can you explain this answer?
Which of the following alkanols would give a positive Lucas test result after 30 minutes of reaction?
a)
1-Butanol
b)
2-Butanol
c)
2-Methyl-2-propanol
d)
1-Propanol
|
|
Deepak Iyer answered |
2-Methyl-2-propanol is a tertiary alkanol, and it will give a positive Lucas test result within 30 minutes due to its high reactivity.
In the Lucas test, which class of alkanol forms an immediate turbidity or precipitate?- a)Primary alkanols
- b)Secondary alkanols
- c)Tertiary alkanols
- d)All alkanols produce the same result
Correct answer is option 'C'. Can you explain this answer?
In the Lucas test, which class of alkanol forms an immediate turbidity or precipitate?
a)
Primary alkanols
b)
Secondary alkanols
c)
Tertiary alkanols
d)
All alkanols produce the same result
|
|
Deepak Iyer answered |
Tertiary alkanols react quickly with the Lucas reagent and form an immediate turbidity or precipitate, indicating their presence.
Chapter doubts & questions for Alkanols - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Alkanols - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup