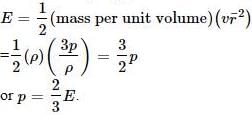All Exams >
JAMB >
Physics for JAMB >
All Questions
All questions of Thermal Expansion, Gas Laws & Kinetic Theory of Gases for JAMB Exam
A steel tape is calibrated at 20° C. A piece of wood is being measured by steel tape at 10°C and reading is 30 cm on the tape. The real length of the wood is:
a)Equal to 30 cmb)less than 30 cmc)cannot be perdictedd)more than 30 cmCorrect answer is option 'B'. Can you explain this answer?
|
|
Preeti Khanna answered |
When heated the length between adjacent markings increases. Hence the actual length will be less than
30 cm.
30 cm.
Value of gas constant, R for one mole of a gas is independent of the- a)Atomicity of the gas
- b)Mass of gas
- c)Distance between two molecules of gas at 273 K
- d)Volume of gas
Correct answer is option 'A'. Can you explain this answer?
Value of gas constant, R for one mole of a gas is independent of the
a)
Atomicity of the gas
b)
Mass of gas
c)
Distance between two molecules of gas at 273 K
d)
Volume of gas

|
Trisha Vashisht answered |
We know that PV=nRT also PM=dRT
So in the equation The value of R depends on P , V , n , T , d , M
except atomicity
so the ans is A
So in the equation The value of R depends on P , V , n , T , d , M
except atomicity
so the ans is A
A region of the earth’s atmosphere contains n molecules (treated as ideal gas molecules) per unit volume. The temperature of air in the region is T. If k represents Boltzmann’s constant and R represents universal gas constant, the pressure of air in the region is- a)RT/n
- b)nRkT
- c)nkT
- d)nT/k
Correct answer is option 'C'. Can you explain this answer?
A region of the earth’s atmosphere contains n molecules (treated as ideal gas molecules) per unit volume. The temperature of air in the region is T. If k represents Boltzmann’s constant and R represents universal gas constant, the pressure of air in the region is
a)
RT/n
b)
nRkT
c)
nkT
d)
nT/k
|
|
Lavanya Menon answered |
PV = nRT
Where n = number of moles = m/NA
So, P = (m/V)(R/NA)T
Also, we know that R/NA = k
So, P = nkT
Where n = number of moles = m/NA
So, P = (m/V)(R/NA)T
Also, we know that R/NA = k
So, P = nkT
In the case of real gases, the equation of state, PV = RT (where P, V and T are respectively the pressure, volume and absolute temperature), is strictly satisfied only if corrections are applied to the measured pressure P and the measured volume V. The corrections for P and V arise respectively due to- a)kinetic energy of molecules and collision of molecules
- b)size of molecules and expansion of the container
- c)expansion of the container and intermolecular attraction
- d)intermolecular attraction and the size of molecules
Correct answer is 'D'. Can you explain this answer?
In the case of real gases, the equation of state, PV = RT (where P, V and T are respectively the pressure, volume and absolute temperature), is strictly satisfied only if corrections are applied to the measured pressure P and the measured volume V. The corrections for P and V arise respectively due to
a)
kinetic energy of molecules and collision of molecules
b)
size of molecules and expansion of the container
c)
expansion of the container and intermolecular attraction
d)
intermolecular attraction and the size of molecules

|
Ashish Mishra answered |
D is correct
The kinetic energy of one mole of an ideal gas is E=3/2 RT. Then Cρ will be- a)0.5 R
- b)1.5 R
- c)2.5 R
- d)2.2 R
Correct answer is option 'C'. Can you explain this answer?
The kinetic energy of one mole of an ideal gas is E=3/2 RT. Then Cρ will be
a)
0.5 R
b)
1.5 R
c)
2.5 R
d)
2.2 R
|
|
Naina Sharma answered |
We know that,
Cp=cv+R
E=3Rt/2
also,
E=Cv
cp=R+3RT/2
=5/2 RT
=2.5R
Cp=cv+R
E=3Rt/2
also,
E=Cv
cp=R+3RT/2
=5/2 RT
=2.5R
When water is heated from 0° C to 20° C its volume:- a)first decreases and then increases
- b)goes on increasing
- c)remains constant up to 15°C and then increases
- d)goes on decreasing
Correct answer is option 'A'. Can you explain this answer?
When water is heated from 0° C to 20° C its volume:
a)
first decreases and then increases
b)
goes on increasing
c)
remains constant up to 15°C and then increases
d)
goes on decreasing
|
|
Pooja Shah answered |
When water is heated from 0°C, its volume decreases because its density increases and you can see this effect upto 4°C. Since the density of ice is maximum at 4°C, afterwards as the density decreases the volume increases. The main reason for this is hydrogen bond in ice gets cleaved due to the melting of ice.
Oxygen and nitrogen in two enclosures have the same mass, volume and pressure. The ratio of the temperature of oxygen to that of nitrogen is:- a)8/7
- b)49/64
- c)1
- d)7/8
Correct answer is option 'A'. Can you explain this answer?
Oxygen and nitrogen in two enclosures have the same mass, volume and pressure. The ratio of the temperature of oxygen to that of nitrogen is:
a)
8/7
b)
49/64
c)
1
d)
7/8
|
|
Neha Joshi answered |
For same mass the ratio of moles of oxygen to that of nitrogen is 14:16 = 7:8
And we know that PV = nRT
Hence as V and P are also same, ratio of temperature of oxygen to that of nitrogen is inverse of the ratio of moles that is 8:7
And we know that PV = nRT
Hence as V and P are also same, ratio of temperature of oxygen to that of nitrogen is inverse of the ratio of moles that is 8:7
Water contract on heating between the temperatures- a)0°C to 4°C
- b)0°K to 4°K
- c)0o K to 273 K
- d)0°F to 4°F
Correct answer is option 'A'. Can you explain this answer?
Water contract on heating between the temperatures
a)
0°C to 4°C
b)
0°K to 4°K
c)
0o K to 273 K
d)
0°F to 4°F
|
|
Geetika Shah answered |
When water is heated from 0oC, its volume decreases because its density increases and you can see this effect upto 4oC. Since the density of ice is maximum at 4oC, afterwards as the density decreases the volume increases. The main reason for this is hydrogen bond in ice gets cleaved due to the melting of ice.
Three moles of an ideal monoatomic gas is initially in the state A shown in the adjoining pressure-temperature graph. It is taken to state B without changing its pressure. If R is the universal gas constant, the work done by the gas in this process is
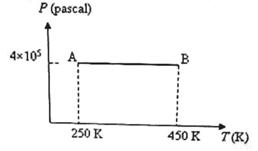
- a)600 R
- b)400 R
- c)300 R
- d)200 R
Correct answer is option 'A'. Can you explain this answer?
Three moles of an ideal monoatomic gas is initially in the state A shown in the adjoining pressure-temperature graph. It is taken to state B without changing its pressure. If R is the universal gas constant, the work done by the gas in this process is


a)
600 R
b)
400 R
c)
300 R
d)
200 R

|
Infinity Academy answered |
The work done by the gas in taking it from state A to state B = PΔV where ΔW is the increase in volume at constant pressure P.
We have PV = μRT where p is the number of moles in the sample of the gas and R is the universal gas constant.
Therefore we have PΔV = μR ΔT = 3 xR(450 - 250) = 600R
Which one of the following quantities can be zero on an average for the molecules of an ideal gas in equilibrium?- a)Speed
- b)Momentum
- c)Kinetic Energy
- d)Density
Correct answer is 'B'. Can you explain this answer?
Which one of the following quantities can be zero on an average for the molecules of an ideal gas in equilibrium?
a)
Speed
b)
Momentum
c)
Kinetic Energy
d)
Density
|
|
Riya Banerjee answered |
In case of ideal gases the average velocity is always zero. Hence the average momentum is zero.
Whereas average speed is non- zero so the kinetic energy is also non-zero, as these two are scalar quantities.
Whereas average speed is non- zero so the kinetic energy is also non-zero, as these two are scalar quantities.
The temperature for which the reading on Celsius and Fahrenheit scales are identical is- a)-273°C, -273 °F
- b)-30°C, -30 °F
- c)0 °C, 0 °F
- d)-40 °C, -40 °F
Correct answer is option 'D'. Can you explain this answer?
The temperature for which the reading on Celsius and Fahrenheit scales are identical is
a)
-273°C, -273 °F
b)
-30°C, -30 °F
c)
0 °C, 0 °F
d)
-40 °C, -40 °F
|
|
Preeti Iyer answered |
The Celsius and Fahrenheit are two important temperature scales. The Fahrenheit scale is used primarily in the United States, while Celsius is used throughout the world. The two scales have different zero points and the Celsius degree is bigger than the Fahrenheit one. There is one point on the Fahrenheit and Celsius scales where the temperatures in degrees are equal. This is -40 degree C and -40 degree F.
Can you explain the answer of this question below:Two identical rectangular strips, one of copper and the other of steel, are riveted as shown to form a bi-metal strip. On heating, the bi-metal strip will

- A:
bend with steel on the concave side
- B:
get twisted
- C:
remain straight
- D:
bend with steel on the convex side
The answer is a.
Two identical rectangular strips, one of copper and the other of steel, are riveted as shown to form a bi-metal strip. On heating, the bi-metal strip will

bend with steel on the concave side
get twisted
remain straight
bend with steel on the convex side
|
|
Nandini Patel answered |
On heating, the copper strip will suffer greater elongation and hence the bimetal strip will bend with the steel strip on the concave side.
[Bimetal strips are widely used in thermal switching applications such as automatic electric iron].
The number of degrees of freedom a diatomic molecule is- a)6
- b)5.0
- c)3
- d)5.3
Correct answer is option 'D'. Can you explain this answer?
The number of degrees of freedom a diatomic molecule is
a)
6
b)
5.0
c)
3
d)
5.3

|
Akshara Chopra answered |
Explanation:Degrees of freedom of a system refers to the possible independent motions a system can have.the total degrees of freedom describing the motion of a diatomic molecule is 5.3 for translation and 2 for rotation
Four moles of an ideal diatomic gas is heated at constant volume from 20° C to 30° C. The molar specific heat of the gas at constant pressure (Cp) is 30.3 Jmol-1K-1 and the universal gas constant (R) is 8.3 Jmol-1K-1. The increase in internal energy of the gas is- a)332 J
- b)80.3 J
- c)303 J
- d)880 J
Correct answer is 'D'. Can you explain this answer?
Four moles of an ideal diatomic gas is heated at constant volume from 20° C to 30° C. The molar specific heat of the gas at constant pressure (Cp) is 30.3 Jmol-1K-1 and the universal gas constant (R) is 8.3 Jmol-1K-1. The increase in internal energy of the gas is
a)
332 J
b)
80.3 J
c)
303 J
d)
880 J
|
|
Raghav Bansal answered |
The value of Cp is 30.3
and as Cp-Cv = R(8.3)
hence Cv = 30.3-8.3
Cv is 22
change in internal energy = no of moles × Cv × change in temperature
hence
change in internal energy = 22 × 4 × 10
= 880j
Hence Option D is correct.
and as Cp-Cv = R(8.3)
hence Cv = 30.3-8.3
Cv is 22
change in internal energy = no of moles × Cv × change in temperature
hence
change in internal energy = 22 × 4 × 10
= 880j
Hence Option D is correct.
Kinetic theory explains the behavior- a)of liquids based on the idea that the liquids consist of rapidly moving atoms or molecules
- b)of gases based on the idea that the gas consists of rapidly moving atoms or molecules
- c)of solids based on the idea that the solid consists of rapidly vibrating atoms or molecules
- d)of solids and liquids based on the idea that they gas consist of rapidly vibrating atoms or molecules
Correct answer is option 'B'. Can you explain this answer?
Kinetic theory explains the behavior
a)
of liquids based on the idea that the liquids consist of rapidly moving atoms or molecules
b)
of gases based on the idea that the gas consists of rapidly moving atoms or molecules
c)
of solids based on the idea that the solid consists of rapidly vibrating atoms or molecules
d)
of solids and liquids based on the idea that they gas consist of rapidly vibrating atoms or molecules
|
|
Shreya Gupta answered |
Kinetic theory explains the behaviour of gases based on the idea that the gas consists of rapidly moving atoms or molecules. This is possible as the inter-atomic forces, which are short range forces that are important for solids and liquids, can be neglected for gases.
Mean free path is the- a)maximum distance between collisions
- b)minimum distance between collisions
- c)average distance between collisions
- d)(maximum distance + minimum distance )/ 2 between collisions
Correct answer is option 'C'. Can you explain this answer?
Mean free path is the
a)
maximum distance between collisions
b)
minimum distance between collisions
c)
average distance between collisions
d)
(maximum distance + minimum distance )/ 2 between collisions

|
Kaavya Mukherjee answered |
Explanation:the mean free path is the average distance traveled by a moving particle (such as an atom, a molecule, a photon) between successive impacts (collisions), which modify its direction or energy or other particle properties.
Which phase of matter has maximum value of temperature coefficient of cubical expansion?- a)Gaseous
- b)Solid
- c)Liquid
- d)none of these
Correct answer is option 'A'. Can you explain this answer?
Which phase of matter has maximum value of temperature coefficient of cubical expansion?
a)
Gaseous
b)
Solid
c)
Liquid
d)
none of these
|
|
Pooja Shah answered |
Gas expands more than solid and liquid because gas particles are far apart hence freely move.
According to kinetic theory of gases, 0K is that temperature at which for an ideal gas- a)pressure is not zero
- b)internal energy is zero
- c)nearly all molecular motion becomes very rapid
- d)volume is not zero
Correct answer is option 'B'. Can you explain this answer?
According to kinetic theory of gases, 0K is that temperature at which for an ideal gas
a)
pressure is not zero
b)
internal energy is zero
c)
nearly all molecular motion becomes very rapid
d)
volume is not zero

|
Sara Saldi answered |
Because at temperature 0K nearly all molecular motion happen to stop. insisting the internal energy to be simple.
I hope it helped
I hope it helped
At absolute zero temperature may be defined as that temperature at which- a)Volume is maximum
- b)Root mean square velocity of the gas molecule reduces to zero
- c)Temperature is 273 K
- d)Mass of molecules of gas is zero
Correct answer is option 'B'. Can you explain this answer?
At absolute zero temperature may be defined as that temperature at which
a)
Volume is maximum
b)
Root mean square velocity of the gas molecule reduces to zero
c)
Temperature is 273 K
d)
Mass of molecules of gas is zero
|
|
Gaurav Kumar answered |
At 0K temperature we know that there is no molecular motion, that is the KE of the particles gets 0. Thus we can say the combined KE of a gaseous system is zero, but as there combined mass cant be zero thus the combined of the square of velocities of the particles is zero, which means that the root mean square velocity of the gas is zero.
When the temperature goes up, the pressure inside a rigid container will _____.- a)remain unchanged
- b)go down
- c)cause particles to cool
- d)go up
Correct answer is option 'D'. Can you explain this answer?
When the temperature goes up, the pressure inside a rigid container will _____.
a)
remain unchanged
b)
go down
c)
cause particles to cool
d)
go up
|
|
Vijay Bansal answered |
The pressure law states that for a constant volume of gas in a sealed container the temperature of the gas is directly proportional to its pressure. This can be easily understood by visualising the particles of gas in the container moving with a greater energy when the temperature is increased.
One mole of hydrogen gas is heated at constant pressure from 300 K to 420 K. Calculate the increase in its internal energy.- a)2.49 kJ
- b)2.65 kJ
- c)2.15 kJ
- d)2.85 kJ
Correct answer is option 'A'. Can you explain this answer?
One mole of hydrogen gas is heated at constant pressure from 300 K to 420 K. Calculate the increase in its internal energy.
a)
2.49 kJ
b)
2.65 kJ
c)
2.15 kJ
d)
2.85 kJ

|
Madhurima Dasgupta answered |
Explanation:
Hydrogen is a diatomic gas. (CV = 2.5R)
Change in internal energy
ΔU = nCVΔT = 1 x 2.5 x 8.31 x 120 = 2493J = 2.49KJ
Calculate the change in internal energy of 3.00 mol of helium gas when its temperature is increased by 2.00 K.- a)85.0 J
- b)95.0 J
- c)65.0 J
- d)75.0 J
Correct answer is option 'D'. Can you explain this answer?
Calculate the change in internal energy of 3.00 mol of helium gas when its temperature is increased by 2.00 K.
a)
85.0 J
b)
95.0 J
c)
65.0 J
d)
75.0 J

|
Krithika Kulkarni answered |
Explanation:
Helium is a monoatomic gas.(CV = 1.5R)
change in internal energy
ΔU=nCVΔT=3×1.5×8.31×2=75J
One mole of hydrogen gas is heated at constant pressure from 300 K to 420 K. Calculate the energy transferred by heat to the gas- a)3.86 kJ
- b)3.49 kJ
- c)3.66 kJ
- d)3.26 kJ
Correct answer is option 'B'. Can you explain this answer?
One mole of hydrogen gas is heated at constant pressure from 300 K to 420 K. Calculate the energy transferred by heat to the gas
a)
3.86 kJ
b)
3.49 kJ
c)
3.66 kJ
d)
3.26 kJ
|
|
Priya Deshpande answered |
To calculate the energy transferred by heat to the gas, we can use the formula:
q = nCΔT
Where:
q = energy transferred by heat
n = number of moles of gas
C = molar heat capacity at constant pressure
ΔT = change in temperature
Given:
n = 1 mole
ΔT = 420 K - 300 K = 120 K
We need to find the value of C, the molar heat capacity at constant pressure.
The molar heat capacity at constant pressure (Cp) for an ideal gas can be calculated using the equation:
Cp = 5/2R
Where:
R = ideal gas constant = 8.314 J/mol·K
Substituting the values, we have:
Cp = 5/2 * 8.314 J/mol·K = 20.785 J/mol·K
Now, we can substitute the values of n, C, and ΔT into the formula:
q = (1 mole)(20.785 J/mol·K)(120 K)
q = 2494.2 J
To convert the energy from joules to kilojoules, we divide by 1000:
q = 2494.2 J / 1000 = 2.4942 kJ
Therefore, the energy transferred by heat to the gas is approximately 2.4942 kJ. None of the given options matches this value, so we need to consider significant figures.
The given temperature values are given to three significant figures (300 K and 420 K). Therefore, our final answer should also be rounded to three significant figures.
Rounding 2.4942 kJ to three significant figures, we get:
2.49 kJ
Among the given options, option B, 3.49 kJ, is the closest match to our rounded answer.
q = nCΔT
Where:
q = energy transferred by heat
n = number of moles of gas
C = molar heat capacity at constant pressure
ΔT = change in temperature
Given:
n = 1 mole
ΔT = 420 K - 300 K = 120 K
We need to find the value of C, the molar heat capacity at constant pressure.
The molar heat capacity at constant pressure (Cp) for an ideal gas can be calculated using the equation:
Cp = 5/2R
Where:
R = ideal gas constant = 8.314 J/mol·K
Substituting the values, we have:
Cp = 5/2 * 8.314 J/mol·K = 20.785 J/mol·K
Now, we can substitute the values of n, C, and ΔT into the formula:
q = (1 mole)(20.785 J/mol·K)(120 K)
q = 2494.2 J
To convert the energy from joules to kilojoules, we divide by 1000:
q = 2494.2 J / 1000 = 2.4942 kJ
Therefore, the energy transferred by heat to the gas is approximately 2.4942 kJ. None of the given options matches this value, so we need to consider significant figures.
The given temperature values are given to three significant figures (300 K and 420 K). Therefore, our final answer should also be rounded to three significant figures.
Rounding 2.4942 kJ to three significant figures, we get:
2.49 kJ
Among the given options, option B, 3.49 kJ, is the closest match to our rounded answer.
The relation between coefficient of volume expansion 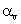 and coefficient of linear expansion
and coefficient of linear expansion 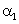 is
is- a)αv = 2α1
- b)α1 = 3αv
- c)αv = 3α1
- d)α1 = 2αv
Correct answer is option 'C'. Can you explain this answer?
The relation between coefficient of volume expansion 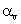 and coefficient of linear expansion
and coefficient of linear expansion 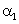 is
is
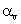 and coefficient of linear expansion
and coefficient of linear expansion 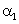 is
isa)
αv = 2α1
b)
α1 = 3αv
c)
αv = 3α1
d)
α1 = 2αv

|
The Amazing Spider Fan answered |
This is simple,because the coefficients of linear expansion A,areal expansion B,and volume expansion G,are in the ratio of 1:2:3 i.e;
A:B:G::1:2:3
Hence,G=3A
In which case are the atoms relatively rigidly fixed?- a)gases
- b)liquids and gases
- c)solids
- d)liquids
Correct answer is option 'C'. Can you explain this answer?
In which case are the atoms relatively rigidly fixed?
a)
gases
b)
liquids and gases
c)
solids
d)
liquids

|
Pranav Saha answered |
Explanation:
Atoms in a gas are well separated with no regular arrangement. Atoms vibrate and move freely at high speeds
Atoms in a liquid are close together with no regular arrangement. Atoms vibrate, move about, and slide past each other.
Atoms in a solid are tightly packed, usually in a regular pattern. Atoms vibrate (jiggle) but generally do not move from place to place.
Kinetic theory relates measurable properties.- a)of liquids such as viscosity, conduction and diffusion with molecular parameters, yielding estimates of molecular sizes and masses
- b)of super cooled liquids such as viscosity, creep and diffusion with molecular parameters, yielding estimates of molecular sizes and masses
- c)of solids such as expansion, conduction and elongation with molecular parameters, yielding estimates of molecular sizes and masses
- d)of gases such as viscosity, conduction and diffusion with molecular parameters, yielding estimates of molecular sizes and masses
Correct answer is option 'D'. Can you explain this answer?
Kinetic theory relates measurable properties.
a)
of liquids such as viscosity, conduction and diffusion with molecular parameters, yielding estimates of molecular sizes and masses
b)
of super cooled liquids such as viscosity, creep and diffusion with molecular parameters, yielding estimates of molecular sizes and masses
c)
of solids such as expansion, conduction and elongation with molecular parameters, yielding estimates of molecular sizes and masses
d)
of gases such as viscosity, conduction and diffusion with molecular parameters, yielding estimates of molecular sizes and masses

|
Moumita Chakraborty answered |
Explanation:Kinetic theory explains the behaviour of gases based on the idea that the gas consists of rapidly moving atoms or molecules. It also relates measurable properties of gases such as viscosity, conduction and diffusion with molecular parameters, yielding estimates of molecular sizes and masses.
One mole of an ideal monatomic gas is at an initial temperature of 300 K. The gas undergoes an isovolumetric process, acquiring 500 J of energy by heat. It then undergoes an isobaric process, losing this same amount of energy by heat. Determine the work done on the gas.- a)123 J
- b)231 J
- c)333 J
- d)200 J
Correct answer is option 'D'. Can you explain this answer?
One mole of an ideal monatomic gas is at an initial temperature of 300 K. The gas undergoes an isovolumetric process, acquiring 500 J of energy by heat. It then undergoes an isobaric process, losing this same amount of energy by heat. Determine the work done on the gas.
a)
123 J
b)
231 J
c)
333 J
d)
200 J

|
Aniket Basu answered |
Explanation:
At const volume,
Q = 500 J
Q=nCPΔT
500=1×2.5×8.31ΔT
ΔT=24.06
W=nRΔT=1×8.31×24.06=200J
Four liters of a diatomic ideal gas ( λ =1.4) confined to a cylinder is subject to a closed cycle. Initially, the gas is at 1.00 atm and at 300 K. First, its pressure is tripled under constant volume. Then, it expands adiabatically to its original pressure. Finally, the gas is compressed isobarically to its original volume. Find the temperature at the end of the cycle- a)276 K
- b)285 K
- c)300 K
- d)332 K
Correct answer is option 'C'. Can you explain this answer?
Four liters of a diatomic ideal gas ( λ
=1.4
) confined to a cylinder is subject to a closed cycle. Initially, the gas is at 1.00 atm and at 300 K. First, its pressure is tripled under constant volume. Then, it expands adiabatically to its original pressure. Finally, the gas is compressed isobarically to its original volume. Find the temperature at the end of the cyclea)
276 K
b)
285 K
c)
300 K
d)
332 K

|
Anshu Joshi answered |
According to Atomic Hypothesis: little particles of atom- a)attract each other when they are at small distance apart, but repel upon being squeezed into one another
- b)repel each other when they are at small distance apart, but attract upon being squeezed into one another
- c)repel each other when they are at small distance apart, but repel upon being squeezed into one another
- d)repel each other when they are at large distance apart, but attract upon being separated from one another
Correct answer is option 'A'. Can you explain this answer?
According to Atomic Hypothesis: little particles of atom
a)
attract each other when they are at small distance apart, but repel upon being squeezed into one another
b)
repel each other when they are at small distance apart, but attract upon being squeezed into one another
c)
repel each other when they are at small distance apart, but repel upon being squeezed into one another
d)
repel each other when they are at large distance apart, but attract upon being separated from one another

|
Saikat Sharma answered |
Explanation:At room temperature (=300K) the noble gases are all in the gas phase, they are banging around and colliding into one another like little pool balls. At this temperature, when the atoms collide they appear to elastically bounce off of one another, but this bounce is actually a result of atomic repulsion. The atoms are traveling so fast and they approach each other so quickly that their momentum 'squeezes' them together until the atomic repulsion pushes them back apart.
Four cylindrical rods of different radii and lengths are used to connect two heat reservoirs at fixed temperatures t1 and t2 respectively. From the following pick out the rod which will conduct the maximum quantity of heat:- a)Radius 1 cm, length 2 m
- b)Radius 1 cm, length 1 m
- c)Radius 2 cm, length 4 m
- d)Radius 3 cm, length 8 m
Correct answer is option 'D'. Can you explain this answer?
Four cylindrical rods of different radii and lengths are used to connect two heat reservoirs at fixed temperatures t1 and t2 respectively. From the following pick out the rod which will conduct the maximum quantity of heat:
a)
Radius 1 cm, length 2 m
b)
Radius 1 cm, length 1 m
c)
Radius 2 cm, length 4 m
d)
Radius 3 cm, length 8 m
|
|
Neha Sharma answered |
The rate of heat transfer is directly proportional to the bisectional surface area of the solid and inversely proportional to its parallel length.
That is heat conduction rate say H ∝ r2
∝ 1/L
I.e ∝ r2/L
Hence the conduction would be
maximum in case in which r2/L ratio is largest.
That is heat conduction rate say H ∝ r2
∝ 1/L
I.e ∝ r2/L
Hence the conduction would be
maximum in case in which r2/L ratio is largest.
The coefficient of liner expansion of a cubical crystal along three mutually perpendicular direction is  and
and  . What is the coefficient of cubical expansion of crystal?
. What is the coefficient of cubical expansion of crystal?- a)α1 + α2 + α3
- b)α1 + α2 - α3
- c)α1 - α2 - α3
- d)(α1 + α2 )/ α3
Correct answer is option 'A'. Can you explain this answer?
The coefficient of liner expansion of a cubical crystal along three mutually perpendicular direction is  and
and  . What is the coefficient of cubical expansion of crystal?
. What is the coefficient of cubical expansion of crystal?
 and
and  . What is the coefficient of cubical expansion of crystal?
. What is the coefficient of cubical expansion of crystal?a)
α1 + α2 + α3
b)
α1 + α2 - α3
c)
α1 - α2 - α3
d)
(α1 + α2 )/ α3
|
|
Neha Joshi answered |
Cubical expansion means there is expansion is length (a1), breadth (a2) and height (a3). Therefore the net coefficient of cubical expansion is α1 + α2 + α3.
1 mole of a monoatomic gas is mixed with 3 moles of a diatomic gas. What is the molecular specific heat of the mixture at constant volume?- a)12.5 J / mol K
- b)18.7 J / mol K
- c)15.2 J / mol K
- d)22.6 J / mol K
Correct answer is option 'B'. Can you explain this answer?
1 mole of a monoatomic gas is mixed with 3 moles of a diatomic gas. What is the molecular specific heat of the mixture at constant volume?
a)
12.5 J / mol K
b)
18.7 J / mol K
c)
15.2 J / mol K
d)
22.6 J / mol K

|
Aravind Saha answered |
Explanation:
for monoatomic gas
from conservation of energy
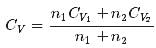
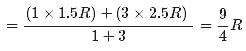
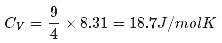
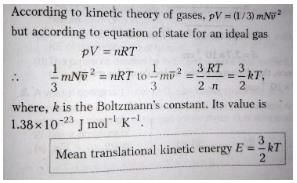
The average kinetic energy of a molecule in an ideal gas is- a)proportional to the pressure
- b)depends on the nature of the ideal gas
- c)proportional to the absolute temperature of the gas
- d)proportional to the volume
Correct answer is option 'C'. Can you explain this answer?
The average kinetic energy of a molecule in an ideal gas is
a)
proportional to the pressure
b)
depends on the nature of the ideal gas
c)
proportional to the absolute temperature of the gas
d)
proportional to the volume
|
|
Priya Patel answered |
SHOW THAT THE AVERAGE TRANSLATIONAL KINETIC ENERGY OF THE MOLECULES OF A GAS IS DIRECTLY PROPORTIONAL TO ABSOLUTE TEMPERATURE. kinetic energy of the molecules of a gas is directly proportional to absolute temperature.
The average kinetic energy of a molecule- a)is proportional to the absolute temperature of the gas
- b)is inversely proportional to the absolute temperature of the gas
- c)is inversely proportional to the molecular mass of the gas
- d)is not dependent on absolute temperature of the gas
Correct answer is option 'A'. Can you explain this answer?
The average kinetic energy of a molecule
a)
is proportional to the absolute temperature of the gas
b)
is inversely proportional to the absolute temperature of the gas
c)
is inversely proportional to the molecular mass of the gas
d)
is not dependent on absolute temperature of the gas

|
Sanika Shaikh answered |
We know formula ,
E/N=(3/2)kbT
the average of kinectic energy per molecules is directly proportional to the absolute temperature of gas.
Three vessels of equal capacity have gases at the same temperature and pressure. The first vessel contains neon (monatomic), the second contains chlorine (diatomic), and the third contains uranium hexafluoride (polyatomic). The number of molecules- a)is the same in all three vessels
- b)is the greatest in the vessel with neon
- c)is the greatest in the vessel with chlorine
- d)is the greatest in the vessel with uranium hexafluoride
Correct answer is option 'A'. Can you explain this answer?
Three vessels of equal capacity have gases at the same temperature and pressure. The first vessel contains neon (monatomic), the second contains chlorine (diatomic), and the third contains uranium hexafluoride (polyatomic). The number of molecules
a)
is the same in all three vessels
b)
is the greatest in the vessel with neon
c)
is the greatest in the vessel with chlorine
d)
is the greatest in the vessel with uranium hexafluoride

|
Sarthak Verma answered |
Explanation:Avogadro's law states that, "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules".
In a 30.0-s interval, 500 hailstones strike a glass window with an area of 0.600 m2 at an angle of 45.0° to the window surface. Each hailstone has a mass of 5.00 g and a speed of 8.00 m/s. If the collisions are elastic, what is the pressure on the window?- a)1.97 Pa
- b)1.57 Pa
- c)1.13 Pa
- d)1.32 Pa
Correct answer is option 'B'. Can you explain this answer?
In a 30.0-s interval, 500 hailstones strike a glass window with an area of 0.600 m2 at an angle of 45.0° to the window surface. Each hailstone has a mass of 5.00 g and a speed of 8.00 m/s. If the collisions are elastic, what is the pressure on the window?
a)
1.97 Pa
b)
1.57 Pa
c)
1.13 Pa
d)
1.32 Pa

|
Kaavya Mukherjee answered |
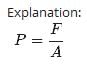
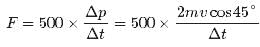
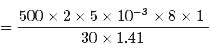
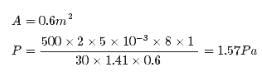
In a mixture of ideal gases at a fixed temperature the heavier molecule has the lower average speed. This is easiest to conclude from the equation- a)
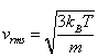
- b)
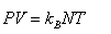
- c)
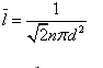
- d)
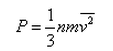
Correct answer is option 'A'. Can you explain this answer?
In a mixture of ideal gases at a fixed temperature the heavier molecule has the lower average speed. This is easiest to conclude from the equation
a)
b)
c)
d)
|
|
Lavanya Menon answered |
In option A, v is inversely proportional to mass. Hence, the condition of the question is seen accurately in that option.
The number of degrees of freedom a monatomic molecule is- a)2
- b)1
- c)3.0
- d)4
Correct answer is option 'C'. Can you explain this answer?
The number of degrees of freedom a monatomic molecule is
a)
2
b)
1
c)
3.0
d)
4
|
|
Pooja Shah answered |
Explanation:Degrees of freedom of a system refers to the possible independent motions a system can have.Monoatomic gas molecule can have 3 independent motion and hence have 3 degrees of freedom (all are translational).
Kinetic theory- a)correctly explains specific heat capacities of many gases
- b)correctly explains specific heat capacities of super cooled liquids
- c)correctly explains specific heat capacities of many solids
- d)correctly explains specific heat capacities of many liquids
Correct answer is option 'A'. Can you explain this answer?
Kinetic theory
a)
correctly explains specific heat capacities of many gases
b)
correctly explains specific heat capacities of super cooled liquids
c)
correctly explains specific heat capacities of many solids
d)
correctly explains specific heat capacities of many liquids
|
|
Ishan Ghosh answered |
Explanation:
Kinetic Theory of Gases and Specific Heat Capacities:
The kinetic theory of gases is a model that describes the behavior of gases based on the motion of their particles. When it comes to specific heat capacities, the kinetic theory of gases correctly explains the specific heat capacities of many gases.
Specific Heat Capacities of Gases:
- According to the kinetic theory of gases, the specific heat capacity of a gas is related to the degrees of freedom of its molecules.
- The specific heat capacity of a gas depends on the translational, rotational, and vibrational motions of its molecules.
- The kinetic theory predicts that for monatomic gases, which have only translational motion, the specific heat capacity at constant volume is 3/2 R, where R is the gas constant.
- For diatomic gases, which can also rotate, the specific heat capacity at constant volume is 5/2 R.
Experimental Evidence:
- Experimental measurements of specific heat capacities of gases have been found to be in good agreement with the predictions of the kinetic theory.
- This provides strong evidence that the kinetic theory of gases correctly explains the specific heat capacities of many gases.
Therefore, option A is correct as the kinetic theory of gases correctly explains the specific heat capacities of many gases based on the motion of their particles.
The molecule of a monatomic gas has only three translational degrees of freedom. Thus, the average energy of a molecule at temperature 'T' is __________.- a)3kBT
- b)(1/3)kBT
- c)(3/2)kBT
- d)(3/4)kBT
Correct answer is option 'C'. Can you explain this answer?
The molecule of a monatomic gas has only three translational degrees of freedom. Thus, the average energy of a molecule at temperature 'T' is __________.
a)
3kBT
b)
(1/3)kBT
c)
(3/2)kBT
d)
(3/4)kBT
|
|
Maulik Chakraborty answered |
Understanding Degrees of Freedom
In thermodynamics, the degrees of freedom of a molecule refer to the number of independent ways in which it can move. For a monatomic gas, the gas molecules can move in three-dimensional space, resulting in three translational degrees of freedom.
Energy and Temperature Relationship
The average energy of a molecule in a gas is related to its temperature through the equipartition theorem. This theorem states that each degree of freedom contributes an average energy of (1/2)kBT, where kB is the Boltzmann constant and T is the absolute temperature.
Calculation of Average Energy
Since a monatomic gas has three translational degrees of freedom, we can calculate the average energy as follows:
- Each degree of freedom contributes (1/2)kBT.
- Therefore, for three degrees of freedom:
- Total average energy = 3 * (1/2)kBT = (3/2)kBT.
Conclusion
Thus, the average energy of a molecule in a monatomic gas at temperature T is:
- (3/2)kBT.
This is why option 'C' is correct. The average energy reflects the kinetic energy associated with the translational motion of the gas molecules. Understanding these principles is crucial for grasping the behavior of gases in thermodynamics.
In thermodynamics, the degrees of freedom of a molecule refer to the number of independent ways in which it can move. For a monatomic gas, the gas molecules can move in three-dimensional space, resulting in three translational degrees of freedom.
Energy and Temperature Relationship
The average energy of a molecule in a gas is related to its temperature through the equipartition theorem. This theorem states that each degree of freedom contributes an average energy of (1/2)kBT, where kB is the Boltzmann constant and T is the absolute temperature.
Calculation of Average Energy
Since a monatomic gas has three translational degrees of freedom, we can calculate the average energy as follows:
- Each degree of freedom contributes (1/2)kBT.
- Therefore, for three degrees of freedom:
- Total average energy = 3 * (1/2)kBT = (3/2)kBT.
Conclusion
Thus, the average energy of a molecule in a monatomic gas at temperature T is:
- (3/2)kBT.
This is why option 'C' is correct. The average energy reflects the kinetic energy associated with the translational motion of the gas molecules. Understanding these principles is crucial for grasping the behavior of gases in thermodynamics.
Estimate the total number of air molecules (inclusive of oxygen, nitrogen, water vapor and other constituents) in a room of capacity 25.0 m3 at a temperature of 270C and 1 atm pressure.- a)6.50 x 1026
- b)6.60 x 1026
- c)6.10 x 1026
- d)6.30 x 1026
Correct answer is option 'C'. Can you explain this answer?
Estimate the total number of air molecules (inclusive of oxygen, nitrogen, water vapor and other constituents) in a room of capacity 25.0 m3 at a temperature of 270C and 1 atm pressure.
a)
6.50 x
1026
b)
6.60 x
1026
c)
6.10 x
1026
d)
6.30 x
1026

|
Aniket Basu answered |
Two moles of an ideal gas (γ=1.4) expands slowly and adiabatically from a pressure of 5.00 atm and a volume of 12.0 L to a final volume of 30.0 L. What is the final pressure of the gas?- a)1.59 atm
- b)1.09 atm
- c)1.19 atm
- d)1.39 atm
Correct answer is option 'D'. Can you explain this answer?
Two moles of an ideal gas (γ=1.4) expands slowly and adiabatically from a pressure of 5.00 atm and a volume of 12.0 L to a final volume of 30.0 L. What is the final pressure of the gas?
a)
1.59 atm
b)
1.09 atm
c)
1.19 atm
d)
1.39 atm

|
Anshu Joshi answered |
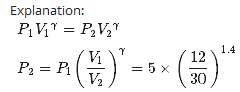
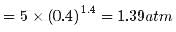
Chapter doubts & questions for Thermal Expansion, Gas Laws & Kinetic Theory of Gases - Physics for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermal Expansion, Gas Laws & Kinetic Theory of Gases - Physics for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Physics for JAMB
259 videos|253 docs|230 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup