All Exams >
EmSAT Achieve >
Physics for EmSAT Achieve >
All Questions
All questions of Fluids Flow for EmSAT Achieve Exam
The angle of contact for the liquid which wets the walls of the vessel is- a)acute
- b)zero
- c)obtuse
- d)900
Correct answer is option 'A'. Can you explain this answer?
The angle of contact for the liquid which wets the walls of the vessel is
a)
acute
b)
zero
c)
obtuse
d)
900
|
|
Anjana Sharma answered |
When liquid molecules are attracted strongly to themselves and weakly to those of solids, it costs lots of energy to create liquid-solid surface and liquid then does not wet the solid.
For Example:
Mercury molecules (which make an obtuse angle with glass) have a strong force of attraction between themselves and a weak force of attraction toward solids. Hence, they tend to form drops.
On the other hand, water molecules make acute angles with glass. They have a weak force of attraction between themselves and a strong force of attraction toward solids. Hence, they tend to spread out.
Water rises to a height of 20 mm in a capillary. If the radius of the capillary is made 1/3 rd of its previous value, to what height will the water now rise in the tube?- a)60 mm
- b)80 mm
- c)40 mm
- d)30 mm
Correct answer is option 'A'. Can you explain this answer?
Water rises to a height of 20 mm in a capillary. If the radius of the capillary is made 1/3 rd of its previous value, to what height will the water now rise in the tube?
a)
60 mm
b)
80 mm
c)
40 mm
d)
30 mm
|
|
Pooja Mehta answered |
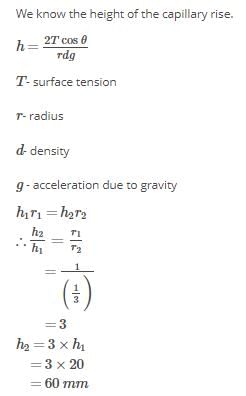
HENCE,CORRECT OPTION IS (A).
The surface tension of a soap solution is 0.05 Nm-1. How much work is done to produce a soap bubble of radius 0.03 m?- a)1.8 x 10-2 J
- b)2.1 x 10-3J
- c)1.5 x 10-2 J
- d)1.1 x 10-3 J
Correct answer is option 'D'. Can you explain this answer?
The surface tension of a soap solution is 0.05 Nm-1. How much work is done to produce a soap bubble of radius 0.03 m?
a)
1.8 x 10-2 J
b)
2.1 x 10-3J
c)
1.5 x 10-2 J
d)
1.1 x 10-3 J
|
|
Suresh Iyer answered |
Work done=total surface x Surface tension
=2x4πr2xσ
=2x4x3.14(0.03)2x 0.05
=1.1x10-3J
=2x4πr2xσ
=2x4x3.14(0.03)2x 0.05
=1.1x10-3J
Which of the following expression is true for surface tension?- a)σ = -F/1
- b)σ = F/1
- c)σ = F.1
- d)σ = F.1.A
Correct answer is option 'B'. Can you explain this answer?
Which of the following expression is true for surface tension?
a)
σ = -F/1
b)
σ = F/1
c)
σ = F.1
d)
σ = F.1.A
|
|
Pooja Shah answered |
The force acting on this line is proportional to the length of this line. If l is the length of imaginary line and F the total force on either side of the line then,
F∝l
⇒ F=Sl
Or, surface tension, S=force/length
From this expression, Surface tension can be defined as the force acting per unit length of an imaginary line drawn on the liquid surface, the direction of force being perpendicular to this line and tangential to the liquid surface.it is denoted by S and it is a scalar quantity.
F∝l
⇒ F=Sl
Or, surface tension, S=force/length
From this expression, Surface tension can be defined as the force acting per unit length of an imaginary line drawn on the liquid surface, the direction of force being perpendicular to this line and tangential to the liquid surface.it is denoted by S and it is a scalar quantity.
When the salt is added to water, the surface tension of liquid mixture will- a)Increase
- b)Depends on the quantity of water
- c)Decrease
- d)Remain unaltered
Correct answer is option 'A'. Can you explain this answer?
When the salt is added to water, the surface tension of liquid mixture will
a)
Increase
b)
Depends on the quantity of water
c)
Decrease
d)
Remain unaltered
|
|
Suresh Reddy answered |
NaCl salts cause an increase of the surface tension and the residence time of interfacial water molecules.
When an air bubble of radius R lies at a depth h below the free surface of a liquid of density ρ and surface tension Sla, then the excess pressure inside the bubble will be- a)
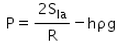
- b)
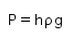
- c)
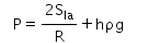
- d)
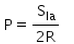
Correct answer is option 'C'. Can you explain this answer?
When an air bubble of radius R lies at a depth h below the free surface of a liquid of density ρ and surface tension Sla, then the excess pressure inside the bubble will be
a)
b)
c)
d)

|
Ambition Institute answered |
Excess pressure inside a cavity or air bubble in liquid formula
P[excess] = P[inside] - P [outside]
P [outside] =P[atm]
P[inside]= P[atm] +hpg [here p represents density] +2T/R
P[excess] = P[atm] +hpg [here p represents density] +2T/R-P[atm]
P[excess] = hpg+2T/R
Now substitute value for T
P[excess] = hpg+2S/R
Hence C is correct.
P[excess] = P[inside] - P [outside]
P [outside] =P[atm]
P[inside]= P[atm] +hpg [here p represents density] +2T/R
P[excess] = P[atm] +hpg [here p represents density] +2T/R-P[atm]
P[excess] = hpg+2T/R
Now substitute value for T
P[excess] = hpg+2S/R
Hence C is correct.
When wetting agents like soap or dyes are added to water, the angle of contact becomes- a)900
- b)600
- c)Large
- d)Small
Correct answer is option 'D'. Can you explain this answer?
When wetting agents like soap or dyes are added to water, the angle of contact becomes
a)
900
b)
600
c)
Large
d)
Small
|
|
Krishna Iyer answered |
When wetting agents like soap or dyes are added to water, the angle of contact becomes small. This happens so that the particles penetrate well and become effective.
The angle of contact for liquid on a solid surface is the angle between:- a)the tangent to the liquid surface at the point of contact and the solid surface
- b)the tangent to the solid surface at the point of contact and the liquid surface
- c)the liquid surface and the solid surface at the point of contact
- d)none of these
Correct answer is option 'A'. Can you explain this answer?
The angle of contact for liquid on a solid surface is the angle between:
a)
the tangent to the liquid surface at the point of contact and the solid surface
b)
the tangent to the solid surface at the point of contact and the liquid surface
c)
the liquid surface and the solid surface at the point of contact
d)
none of these
|
|
Raghav Bansal answered |
The angle of contact for liquid on a solid surface is the angle between the tangent to the liquid surface at the point of contact and the solid surface. This is the definition of angle of contact.
Angle of contact of water proofing agent are generally- a)large
- b)small
- c)less then 200
- d)Can be either large or small
Correct answer is option 'A'. Can you explain this answer?
Angle of contact of water proofing agent are generally
a)
large
b)
small
c)
less then 200
d)
Can be either large or small
|
|
Jayant Mishra answered |
Generally, if the water contact angle is smaller than 90DEG, the solid surface is considered hydrophilic and if the water contact angle is larger than 90DEG, the solid surface is considered hydrophobic.
The liquid that does not wet the solid surface has an ______ angle of contact.- a)900
- b)acute
- c)obtuse
- d)600
Correct answer is option 'C'. Can you explain this answer?
The liquid that does not wet the solid surface has an ______ angle of contact.
a)
900
b)
acute
c)
obtuse
d)
600

|
Aashika Packiaselvam answered |
The angle should be obtuse.... cuz at this angle, the liquid surface moves away from the solid surface and hence cannot wet the solid.
When impurity is added to a liquid, its surface tension- a)decreases
- b)first decreases and then increases
- c)increases
- d)remains same
Correct answer is option 'A'. Can you explain this answer?
When impurity is added to a liquid, its surface tension
a)
decreases
b)
first decreases and then increases
c)
increases
d)
remains same
|
|
Arun Khanna answered |
When impurities are mixed in liquid, surface tension of liquid decreases. The soluble substances when dissolved in water, decreases the surface tension of water.
When a capillary tube of radius r is dipped in a liquid of density ρ and surface tension S, the liquid rises or falls through a distance- a)
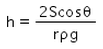
- b)
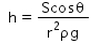
- c)
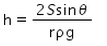
- d)
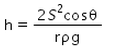
Correct answer is option 'A'. Can you explain this answer?
When a capillary tube of radius r is dipped in a liquid of density ρ and surface tension S, the liquid rises or falls through a distance
a)
b)
c)
d)

|
Ambition Institute answered |
As we know,
S=rhdg/2cosθ
⇒ Pressure difference =hdg=2Scosθ/r
The property by virtue of which the free surface of a liquid at rest behaves like an elastic stretched membrane tending to contract so as to occupy minimum surface area is known as- a)Bernoulli’s principle
- b)Surface tension
- c)Surface energy
- d)Viscosity
Correct answer is option 'B'. Can you explain this answer?
The property by virtue of which the free surface of a liquid at rest behaves like an elastic stretched membrane tending to contract so as to occupy minimum surface area is known as
a)
Bernoulli’s principle
b)
Surface tension
c)
Surface energy
d)
Viscosity
|
|
Naina Sharma answered |
The property by virtue of which the free surface of a liquid at rest behaves like an elastic stretched membrane tending to contract so as to occupy minimum surface area is known as surface tension. By the definition of surface tension.
By which phenomenon does the water rise from roots to leaves of plants?- a)Capillary action
- b)Surface Tension
- c)Bernoulli’s Theorem
- d)Viscosity
Correct answer is option 'A'. Can you explain this answer?
By which phenomenon does the water rise from roots to leaves of plants?
a)
Capillary action
b)
Surface Tension
c)
Bernoulli’s Theorem
d)
Viscosity
|
|
Arun Khanna answered |
Plants use capillary action to bring water from the soil up through capillaries, small tubes in the plants, to the rest of the plant. Due to gravity, capillary action is not strong enough for the water to travel to the top of the plant. Another process, transpiration pull does the rest of the work.
The force required to take away a flat plate of radius 4 cm from the surface of water is (surface tension of water = 70 dyne/cm)- a)1589. 2 dyne/cm
- b)1645.3 dyne/cm
- c)1758.4 dyne/cm
- d)1221.2 dyne/cm
Correct answer is option 'C'. Can you explain this answer?
The force required to take away a flat plate of radius 4 cm from the surface of water is (surface tension of water = 70 dyne/cm)
a)
1589. 2 dyne/cm
b)
1645.3 dyne/cm
c)
1758.4 dyne/cm
d)
1221.2 dyne/cm
|
|
Mira Sharma answered |
Force due to surface tension acts all along the circumference of the circular plate. Therefore, force required to take away of plate is,
F = T x 2πr = 70 x 2 x 3.14 x 4 = 1758.4 dyne/cm
If drops and bubbles do not collapse under the effect of gravity, it indicates that- a)pressure inside the drop is greater than outside
- b)pressure inside the drop is lower than outside it
- c)Surface tension is low
- d)Viscosity is large
Correct answer is option 'A'. Can you explain this answer?
If drops and bubbles do not collapse under the effect of gravity, it indicates that
a)
pressure inside the drop is greater than outside
b)
pressure inside the drop is lower than outside it
c)
Surface tension is low
d)
Viscosity is large

|
Bhawna Mehta answered |
If drops and bubbles do not collapse under the effect of gravity, it indicates that the pressure inside the drop is greater than the pressure outside. The greater inner pressure prevents the drop from collapsing.
The excess pressure inside a soap bubble is (Here, Sla is the surface tension between the liquid-air interface).- a)
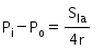
- b)
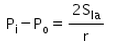
- c)
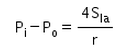
- d)
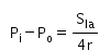
Correct answer is option 'C'. Can you explain this answer?
The excess pressure inside a soap bubble is (Here, Sla is the surface tension between the liquid-air interface).
a)
b)
c)
d)
|
|
Neha Joshi answered |
Excess pressure in water bubble = 2T/r (due to 1 surface only)
Excess pressure in a soap bubble = 4T/r (due to 2 surfaces)
Excess pressure in a soap bubble = 4T/r (due to 2 surfaces)
SI unit of surface tension is- a)N.m2
- b)N.m
- c)N/m
- d)N/m2
Correct answer is option 'C'. Can you explain this answer?
SI unit of surface tension is
a)
N.m2
b)
N.m
c)
N/m
d)
N/m2

|
Supriya Senapati answered |
Surface tension is defined as the ratio of surface force applied on a liquid to the length along which the force acts. so it is newton/meter
The pressure inside a soap bubble of radius R and surface tension S is
- a)2S/R
- b)4S/R
- c)4S.R
- d)2S/R
Correct answer is option 'B'. Can you explain this answer?
The pressure inside a soap bubble of radius R and surface tension S is
a)
2S/R
b)
4S/R
c)
4S.R
d)
2S/R
|
|
Nandini Patel answered |
If R is the radius of a soap bubble and S its surface tension, then the excess pressure inside is 4S/R.
Chapter doubts & questions for Fluids Flow - Physics for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Fluids Flow - Physics for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Physics for EmSAT Achieve
208 videos|329 docs|212 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup