All Exams >
EmSAT Achieve >
Physics for EmSAT Achieve >
All Questions
All questions of Photoelectric Effect for EmSAT Achieve Exam
The energy of the incident photon is 20 eV and the work function of the photosensitive metal is 10 eV. What is the stopping potential?- a)30 V
- b)5 V
- c)10 V
- d)15 V
Correct answer is option 'C'. Can you explain this answer?
The energy of the incident photon is 20 eV and the work function of the photosensitive metal is 10 eV. What is the stopping potential?
a)
30 V
b)
5 V
c)
10 V
d)
15 V
|
|
Krishna Iyer answered |
Stopping potential (Vo) is given by
Vo=W/q where W is the work function and q are the charge of an electron.
Given W=20eV−10eV=10eV. Also, q=e
Hence, Vo=(10eV)/e=10V
Vo=W/q where W is the work function and q are the charge of an electron.
Given W=20eV−10eV=10eV. Also, q=e
Hence, Vo=(10eV)/e=10V
Photons of energy 6 eV are incident on a potassium surface of a work function 2.1 eV. What is the stopping potential?- a)-3.9V
- b)-8.1V
- c)-5V
- d)-1.9V
Correct answer is 'A'. Can you explain this answer?
Photons of energy 6 eV are incident on a potassium surface of a work function 2.1 eV. What is the stopping potential?
a)
-3.9V
b)
-8.1V
c)
-5V
d)
-1.9V
|
|
Priyanka Sharma answered |
From photo-electric equation, eV0= E−φ
eV0=(6−2.1)eV
V0= 3.9 V
stopping potential is a negative potential to stop e- at saturated current .
eV0=(6−2.1)eV
V0= 3.9 V
stopping potential is a negative potential to stop e- at saturated current .
Light of frequency 1.5 times the threshold frequency is incident on a photosensitive material .If the frequency is halved and the intensity is doubled, the photoelectric current becomes- a)Doubled
- b)Quadrupled
- c)Halved
- d)zero
Correct answer is option 'D'. Can you explain this answer?
Light of frequency 1.5 times the threshold frequency is incident on a photosensitive material .If the frequency is halved and the intensity is doubled, the photoelectric current becomes
a)
Doubled
b)
Quadrupled
c)
Halved
d)
zero

|
Ayush Joshi answered |
If the frequency is halved and intensity is doubled, the frequency of incident light will become 15/2 = 0.75 times the threshold frequency. So, as ν<νo Hence, photoelectric current will be zero.
A photo-sensitive material would emit electrons, if excited by photons beyond a threshold. To overcome the threshold, one would increase the:- a) voltage applied to the light source
- b) intensity of light
- c) wavelength of light
- d) frequency of light
Correct answer is option 'D'. Can you explain this answer?
A photo-sensitive material would emit electrons, if excited by photons beyond a threshold. To overcome the threshold, one would increase the:
a)
voltage applied to the light source
b)
intensity of light
c)
wavelength of light
d)
frequency of light
|
|
Rohan Singh answered |
The emission of photoelectron takes place only, when the frequency of the incident light is above a certain critical value, characteristic of that metal. The critical value of frequency is known as the threshold frequency for the metal of the emitting electrode.
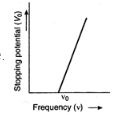
Suppose that when light of certain frequency is incident over a metal surface, the photo- electrons are emitted. To take photoelectric current zero, a particular value of stopping potential will be needed. If we go on reducing the frequency of incident light, the value of stopping potential will also go on decreasing. At certain value of frequency v0, the photoelectric current will become zero, even when no retarding potential is applied. This frequency vq corresponds to the threshold for the metal surface. The emission of photoelectrons does not take place, till frequency of incident light is below this value.

Can you explain the answer of this question below:Light from a bulb is falling on a wooden table but no photo electrons are emitted as
- A:
Work function of wood is less
- B:
Work function of wood is more
- C:
It depends on the frequency
- D:
It is independent of work function
The answer is b.
Light from a bulb is falling on a wooden table but no photo electrons are emitted as
Work function of wood is less
Work function of wood is more
It depends on the frequency
It is independent of work function
|
|
Rishika Patel answered |
Explanation:
When light falls on a metal surface, electrons may be emitted from the metal surface. This phenomenon is known as the photoelectric effect. The electrons emitted from the metal surface are called photoelectrons.
The photoelectric effect can be explained by considering that light is made up of photons. Each photon has a certain amount of energy, given by its frequency. When a photon strikes a metal surface, its energy can be transferred to an electron in the metal. If the energy of the photon is greater than the work function of the metal, the electron can be emitted from the metal surface.
In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it. Therefore, no photoelectrons are emitted. This is because the energy of the photons is not enough to overcome the work function of the wood.
Key Points:
- The photoelectric effect is the emission of electrons from a metal surface when light falls on it.
- The energy of a photon is given by its frequency.
- If the energy of a photon is greater than the work function of the metal, electrons can be emitted from the metal surface.
- In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it, so no photoelectrons are emitted.
When light falls on a metal surface, electrons may be emitted from the metal surface. This phenomenon is known as the photoelectric effect. The electrons emitted from the metal surface are called photoelectrons.
The photoelectric effect can be explained by considering that light is made up of photons. Each photon has a certain amount of energy, given by its frequency. When a photon strikes a metal surface, its energy can be transferred to an electron in the metal. If the energy of the photon is greater than the work function of the metal, the electron can be emitted from the metal surface.
In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it. Therefore, no photoelectrons are emitted. This is because the energy of the photons is not enough to overcome the work function of the wood.
Key Points:
- The photoelectric effect is the emission of electrons from a metal surface when light falls on it.
- The energy of a photon is given by its frequency.
- If the energy of a photon is greater than the work function of the metal, electrons can be emitted from the metal surface.
- In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it, so no photoelectrons are emitted.
Sodium surface is illuminated by ultraviolet and visible radiation successively and the stopping potential determined. The stopping potential is- a)Greater with visible light
- b)Equal in both cases
- c)Greater with ultraviolet light
- d)Infinite in both cases
Correct answer is 'C'. Can you explain this answer?
Sodium surface is illuminated by ultraviolet and visible radiation successively and the stopping potential determined. The stopping potential is
a)
Greater with visible light
b)
Equal in both cases
c)
Greater with ultraviolet light
d)
Infinite in both cases
|
|
Riya Banerjee answered |
λ for U.V is less than λ for visible light
ν for U.V is greater than ν for visible light
∴ potential is greater for U.V light.
as K.Eα1/λ
ν for U.V is greater than ν for visible light
∴ potential is greater for U.V light.
as K.Eα1/λ
A parallel beam of uniform, monochromatic light of wavelength 2640 A has an intensity of 200 W/m2. The number of photons in 1 mm3 of this radiation are ...............
Correct answer is '885'. Can you explain this answer?
A parallel beam of uniform, monochromatic light of wavelength 2640 A has an intensity of 200 W/m2. The number of photons in 1 mm3 of this radiation are ...............
|
|
Rajesh Gupta answered |
I=nhv/ΔtA=[N.h(C/ λ)]/(1mm/c)x(1mm2)
N=200x10-9x2640x10-10/(3x108)2x6.63x10-34
=885 Photons/mm3
N=200x10-9x2640x10-10/(3x108)2x6.63x10-34
=885 Photons/mm3
Light of wavelength 4000 Â is incident on a metal of work function 3.2 x 10-19 J. What is the maximum kinetic energy of the emitted electron?
- a)2.2 x 10-19 J
- b)1.75 x 10-19 J
- c)0.75 x 10-19 J
- d)1.1 x 10-19 J
Correct answer is option 'B'. Can you explain this answer?
Light of wavelength 4000 Â is incident on a metal of work function 3.2 x 10-19 J. What is the maximum kinetic energy of the emitted electron?
a)
2.2 x 10-19 J
b)
1.75 x 10-19 J
c)
0.75 x 10-19 J
d)
1.1 x 10-19 J
|
|
Om Desai answered |
Given,
λ= 4000 Å ;W=3.2× 10-19 j
as we know that
K.E = (hc/ λ) - W
= (6.6× 10-34 × 3 × 108/ 4000×10-10 )- 3.20× 10-19
= 1.75 × 10-19
λ= 4000 Å ;W=3.2× 10-19 j
as we know that
K.E = (hc/ λ) - W
= (6.6× 10-34 × 3 × 108/ 4000×10-10 )- 3.20× 10-19
= 1.75 × 10-19
The electron in a hydrogen atom makes a transition n1 ¾→ n2, where n1 & n2 are the principal quantum numbers of the two states. Assume the Bohr model to be valid. The time period of the electron in the initial state is eight times that in the final state. The possible values of n1 & n2 are :- a) n1 = 4, n2 = 2
- b)n1 = 8, n2 = 2
- c)n1 = 8, n2 = 1
- d)n1 = 6, n2 = 3
Correct answer is option 'A,D'. Can you explain this answer?
The electron in a hydrogen atom makes a transition n1 ¾→ n2, where n1 & n2 are the principal quantum numbers of the two states. Assume the Bohr model to be valid. The time period of the electron in the initial state is eight times that in the final state. The possible values of n1 & n2 are :
a)
n1 = 4, n2 = 2
b)
n1 = 8, n2 = 2
c)
n1 = 8, n2 = 1
d)
n1 = 6, n2 = 3
|
|
Om Desai answered |
For an electron revolving in nth orbit around the nucleus of hydrogen atom, Fcentrifugal=Fcoulomb
Thus, mvn2/ rn=e2/4πϵorn2
Also, mvnrn=nh/2π
On solving these two we get: vn= e21/2ϵohn and rn=( ϵoh2/πme2 ) n2
Time period, T=2πrn/vn⟹T∝n3
Thus, 8T/T=(n1/n2)3⟹n1=2n2
Thus, n1=4,n2=2 and n1=6,n2=3
Thus, mvn2/ rn=e2/4πϵorn2
Also, mvnrn=nh/2π
On solving these two we get: vn= e21/2ϵohn and rn=( ϵoh2/πme2 ) n2
Time period, T=2πrn/vn⟹T∝n3
Thus, 8T/T=(n1/n2)3⟹n1=2n2
Thus, n1=4,n2=2 and n1=6,n2=3
In the experiment on photoelectric effect using light having frequency greater than the threshold frequency, the photocurrent will certainly increase when- a)Anode voltage is incrased
- b)Area of cathode surface is increased
- c)Intensity of incident light is increased
- d)Distance between anode and cathode is increased.
Correct answer is option 'B,C'. Can you explain this answer?
In the experiment on photoelectric effect using light having frequency greater than the threshold frequency, the photocurrent will certainly increase when
a)
Anode voltage is incrased
b)
Area of cathode surface is increased
c)
Intensity of incident light is increased
d)
Distance between anode and cathode is increased.
|
|
Riya Banerjee answered |
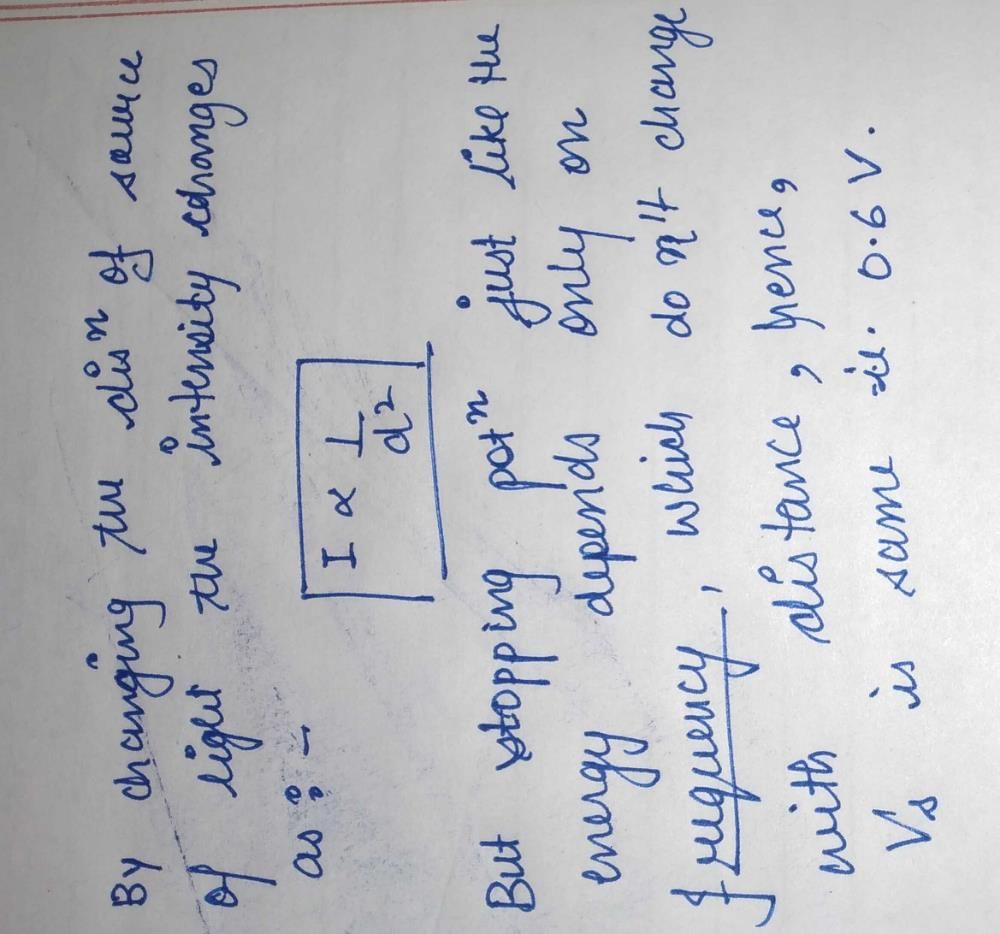
If light of certain frequency, greater than the threshold frequency, is incident of the metal surface the photoelectric current obtained is directly proportional to the number of photo-electrons emitted. On increasing the frequency of incident light (or) by increasing the anode potential the speed of photo-electrons emitted changes but they have no effect on the number of electrons emitted. But by increasing the intensity of the incident light more photo-electrons will be emitted and thereby increasing the photoelectric current.
Also, if we increase the area on which the light is incident we can get more electrons emitted which increases the photoelectric current.
Also, if we increase the area on which the light is incident we can get more electrons emitted which increases the photoelectric current.
When a monochromatic point source of light is at a distance of 0.2 m from a photoelectric cell, the cut off voltage and the saturation current are respectively 0.6 volt and 18.0 mA. If the same source is placed 0.6 m away from the photoelectric cell, then findQ. the saturation current
Correct answer is '2.0'. Can you explain this answer?
When a monochromatic point source of light is at a distance of 0.2 m from a photoelectric cell, the cut off voltage and the saturation current are respectively 0.6 volt and 18.0 mA. If the same source is placed 0.6 m away from the photoelectric cell, then find
Q.
the saturation current
|
|
Neha Sharma answered |
Intensity of photons ↑, no. of electron ↑
The cut off voltage does not depend on distance between cell and source.
But intensity is inversely proportional to square of it's distance.
∴ at d=0.6m,
Cut off voltage =0.6V
but
Is=18×(0.2/0.6)2
=2mA
So, the answers are option 2.0 mA
The cut off voltage does not depend on distance between cell and source.
But intensity is inversely proportional to square of it's distance.
∴ at d=0.6m,
Cut off voltage =0.6V
but
Is=18×(0.2/0.6)2
=2mA
So, the answers are option 2.0 mA
The total energy of the electron in the hydrogen atom in the ground state is -13.6 eV. Which of the following is its kinetic energy in the first excited state?- a)13.6 eV
- b)6.8 eV
- c)3.4 eV
- d)1.825 eV
Correct answer is option 'C'. Can you explain this answer?
The total energy of the electron in the hydrogen atom in the ground state is -13.6 eV. Which of the following is its kinetic energy in the first excited state?
a)
13.6 eV
b)
6.8 eV
c)
3.4 eV
d)
1.825 eV
|
|
Suresh Iyer answered |
We know that,
∣P.E∣/2=∣T.E∣=K.E
So, ∣P.E∣/2=3.4=K.E
So, kinetic energy K.E=3.4 eV
∣P.E∣/2=∣T.E∣=K.E
So, ∣P.E∣/2=3.4=K.E
So, kinetic energy K.E=3.4 eV
Light of two different frequencies whose photons have energies of 1eV and 2.5 eV respectively successively illuminate a metal of work function 0.5eV. The ratio of maximum speed of emitted electrons is- a)1:5
- b)1:4
- c)1:3
- d)1:2
Correct answer is option 'D'. Can you explain this answer?
Light of two different frequencies whose photons have energies of 1eV and 2.5 eV respectively successively illuminate a metal of work function 0.5eV. The ratio of maximum speed of emitted electrons is
a)
1:5
b)
1:4
c)
1:3
d)
1:2
|
|
Shreya Gupta answered |
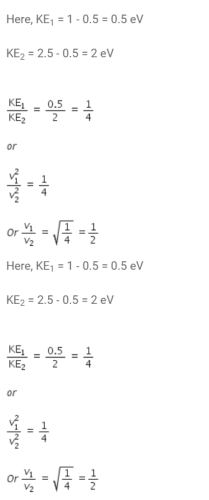
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed to 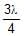 the kinetic energy of the fastest emitted electron will be :
the kinetic energy of the fastest emitted electron will be :- a)3K/4
- b)4K/3
- c)Less than 4K/3
- d)Greater than 4K/3
Correct answer is option 'D'. Can you explain this answer?
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed to 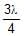 the kinetic energy of the fastest emitted electron will be :
the kinetic energy of the fastest emitted electron will be :
a)
3K/4
b)
4K/3
c)
Less than 4K/3
d)
Greater than 4K/3
|
|
Lavanya Menon answered |
From E=W0+(1/2)mvmax2⇒vmax= √[(2E/m)−(2W0/m)]
where E= hc/λ
If wavelength of incident light charges from λ to 3λ/4 (Decreases)
Let energy of incident light charges from E and speed of fastest electron changes from v to v′ then
v′=√[(2E′/m)−(2W0/m)]
As E∝1/λ⇒E′(4/3)E
Hence v′=√[(2(4/3)E/m)−(2W0/m)]
⇒v′=(4/3)1/2 [(2E/m)− (2W0/m(4/3)1/2)]
So, v′>(4/3)1/2v
where E= hc/λ
If wavelength of incident light charges from λ to 3λ/4 (Decreases)
Let energy of incident light charges from E and speed of fastest electron changes from v to v′ then
v′=√[(2E′/m)−(2W0/m)]
As E∝1/λ⇒E′(4/3)E
Hence v′=√[(2(4/3)E/m)−(2W0/m)]
⇒v′=(4/3)1/2 [(2E/m)− (2W0/m(4/3)1/2)]
So, v′>(4/3)1/2v
An electron is in an excited state in hydrogen-like atom. It has a total energy of –3.4 eV. If the kinetic energy of the electron is E and its de-Broglie wavelength is l, then- a)E = 6.8 eV, l = 6.6 × 10–10 m
- b)E = 3.4 eV, l = 6.6 × 10–10 m
- c)E = 3.4 eV, l = 6.6 × 10–11 m
- d)E = 6.8 eV, l = 6.6 × 10–11 m
Correct answer is option 'B'. Can you explain this answer?
An electron is in an excited state in hydrogen-like atom. It has a total energy of –3.4 eV. If the kinetic energy of the electron is E and its de-Broglie wavelength is l, then
a)
E = 6.8 eV, l = 6.6 × 10–10 m
b)
E = 3.4 eV, l = 6.6 × 10–10 m
c)
E = 3.4 eV, l = 6.6 × 10–11 m
d)
E = 6.8 eV, l = 6.6 × 10–11 m
|
|
Hansa Sharma answered |
The potential energy of an electron is twice the kinetic energy(with negative sign) of an electron.
PE=2E
The total energy is: TE=PE+KE
−3.4=−2×3.4+KE
KE=3.4eV
Let p be the momentum of an electron and m be the mass of an electron.
E=p2/2m
p=√2mE
Now, the De-Broglie wavelength associated with an electron is:
λ=h/p
λ=h/√2mE
λ=6.6×1034/√2×9.1×10−31×(−3.4)×1.6×10−19
λ=6.6×1034/9.95×10−25
0λ=0.66×10−9
λ=6.6×10−10m
PE=2E
The total energy is: TE=PE+KE
−3.4=−2×3.4+KE
KE=3.4eV
Let p be the momentum of an electron and m be the mass of an electron.
E=p2/2m
p=√2mE
Now, the De-Broglie wavelength associated with an electron is:
λ=h/p
λ=h/√2mE
λ=6.6×1034/√2×9.1×10−31×(−3.4)×1.6×10−19
λ=6.6×1034/9.95×10−25
0λ=0.66×10−9
λ=6.6×10−10m
A particular hydrogen like atom has its ground state binding "energy 122.4 eV. Its is in ground state. Then :- a)Its atomic number is 3
- b) An electron of 90eV can excite it.
- c)An electron of kinetic energy nearly 91.8 eV can be brought to almost rest by this atom
- d)An electron of kinetic energy 2.6 eV may emerge from the atom when electron of kinetic energy 125 eV collides with this atom.
Correct answer is option 'A,C,D'. Can you explain this answer?
A particular hydrogen like atom has its ground state binding "energy 122.4 eV. Its is in ground state. Then :
a)
Its atomic number is 3
b)
An electron of 90eV can excite it.
c)
An electron of kinetic energy nearly 91.8 eV can be brought to almost rest by this atom
d)
An electron of kinetic energy 2.6 eV may emerge from the atom when electron of kinetic energy 125 eV collides with this atom.
|
|
Nikita Singh answered |
En= −z213.6eV/ n2
for ground state, n=1
−122.4=−Z213.6eV
When a proton interacts that
EP=hV=E1−E0=Z213.6eV[(1/12)−(1/22)]
=9×13.6eV [3/4]
=91.8eV
So the Atomic number is 3 , a photon of 91.8eV and an electron of 8.2eV are emitted when 100eV electron interacts e− interact with this atom.
for ground state, n=1
−122.4=−Z213.6eV
When a proton interacts that
EP=hV=E1−E0=Z213.6eV[(1/12)−(1/22)]
=9×13.6eV [3/4]
=91.8eV
So the Atomic number is 3 , a photon of 91.8eV and an electron of 8.2eV are emitted when 100eV electron interacts e− interact with this atom.
When a monochromatic point source of light is at a distance of 0.2 m from a photoelectric cell, the cut off voltage and the saturation current are respectively 0.6 volt and 18.0 mA. If the same source is placed 0.6 m away from the photoelectric cell, then findQthe stopping potential
Correct answer is between '0.6,2.0'. Can you explain this answer?
When a monochromatic point source of light is at a distance of 0.2 m from a photoelectric cell, the cut off voltage and the saturation current are respectively 0.6 volt and 18.0 mA. If the same source is placed 0.6 m away from the photoelectric cell, then find
Q
the stopping potential

|
Yt Gamers answered |
663 mW of light from a 540 nm source is incident on the surface of a metal. If only 1 of each 5×109 incident photons in absorbed and causes an electron to be ejected from the surface, the total photocurrent in the circuit is ____________________.- a)5.76 x 1011
- b)5.76 x 109
- c)5.76 x 1010
- d)5.76 x 1012
Correct answer is option 'A'. Can you explain this answer?
663 mW of light from a 540 nm source is incident on the surface of a metal. If only 1 of each 5×109 incident photons in absorbed and causes an electron to be ejected from the surface, the total photocurrent in the circuit is ____________________.
a)
5.76 x 1011
b)
5.76 x 10
9
c)
5.76 x 1010
d)
5.76 x 1012
|
|
Suresh Iyer answered |
N / Δt = no. of photon incident per second.
∴ 663x10-3=(N/Δt)x(hc/λ)
∴N/ Δt=663x10-3/(hc/λ)= 663x10-3/(1242nmeV/540)
(n/Δt)/(N/Δt)=1/(5x109)
n/Δt=[1/(5x109)]x (N/Δt)
=[1/(5x109)]x ((663x10-3x540)/1242nmeV)
I=ne/Δt=5.76x1011A
∴ 663x10-3=(N/Δt)x(hc/λ)
∴N/ Δt=663x10-3/(hc/λ)= 663x10-3/(1242nmeV/540)
(n/Δt)/(N/Δt)=1/(5x109)
n/Δt=[1/(5x109)]x (N/Δt)
=[1/(5x109)]x ((663x10-3x540)/1242nmeV)
I=ne/Δt=5.76x1011A
Two electrons are moving with the same speed v. One electron enters a region of uniform electric field while the other enters a region of uniform magnetic field, then after sometime if the de-Broglie wavelengths of the two are l1 and l2 then :- a)l1 = l2
- b)l1 > l2
- c)l1 < l2
- d) l1 > l2 or l1 < l2
Correct answer is option 'D'. Can you explain this answer?
Two electrons are moving with the same speed v. One electron enters a region of uniform electric field while the other enters a region of uniform magnetic field, then after sometime if the de-Broglie wavelengths of the two are l1 and l2 then :
a)
l1 = l2
b)
l1 > l2
c)
l1 < l2
d)
l1 > l2 or l1 < l2
|
|
Ankita Datta answered |
We know that the de-Broglie wavelength of an electron is given by λ = h/p, where h is the Planck's constant and p is the momentum of the electron.
In the region of uniform electric field, the electron experiences a force in the direction of the electric field and its momentum changes. However, the magnitude of the momentum remains the same as the speed of the electron remains constant. Therefore, the de-Broglie wavelength of the electron does not change and remains l1.
In the region of uniform magnetic field, the electron experiences a force perpendicular to its velocity due to the Lorentz force. This force causes the electron to move in a circular path with a radius given by r = mv/qB, where m is the mass of the electron, v is its velocity, q is its charge and B is the magnetic field strength. As the electron moves in a circular path, its momentum changes direction continuously. Therefore, the de-Broglie wavelength of the electron changes and is given by λ2 = h/(mv/qB) = hqB/mv.
Since the two electrons have the same speed, their momenta are the same. Therefore, we can write:
λ2 = hqB/mv = (h/mv) * (qB)
Comparing this with the expression for λ1, we can see that:
λ2/λ1 = (h/mv) * (qB)/(h/mv) = qB/v
Therefore, the ratio of the de-Broglie wavelengths of the two electrons depends on the ratio of the magnetic field strength to the speed of the electron. Hence, option (c) is the correct answer.
In the region of uniform electric field, the electron experiences a force in the direction of the electric field and its momentum changes. However, the magnitude of the momentum remains the same as the speed of the electron remains constant. Therefore, the de-Broglie wavelength of the electron does not change and remains l1.
In the region of uniform magnetic field, the electron experiences a force perpendicular to its velocity due to the Lorentz force. This force causes the electron to move in a circular path with a radius given by r = mv/qB, where m is the mass of the electron, v is its velocity, q is its charge and B is the magnetic field strength. As the electron moves in a circular path, its momentum changes direction continuously. Therefore, the de-Broglie wavelength of the electron changes and is given by λ2 = h/(mv/qB) = hqB/mv.
Since the two electrons have the same speed, their momenta are the same. Therefore, we can write:
λ2 = hqB/mv = (h/mv) * (qB)
Comparing this with the expression for λ1, we can see that:
λ2/λ1 = (h/mv) * (qB)/(h/mv) = qB/v
Therefore, the ratio of the de-Broglie wavelengths of the two electrons depends on the ratio of the magnetic field strength to the speed of the electron. Hence, option (c) is the correct answer.
Statement-1: In the process of photo electric emission, all the emitted photoelectrons have same K.E.
Statement-2: According to einstein's photo electric equation KEmax = hn – f.
- a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.
- b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.
- c)Statement-1 is true, statement-2 is false.
- d)Statement-1 is false, statement-2 is true.
Correct answer is option 'D'. Can you explain this answer?
Statement-1: In the process of photo electric emission, all the emitted photoelectrons have same K.E.
Statement-2: According to einstein's photo electric equation KEmax = hn – f.
a)
Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.
b)
Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.
c)
Statement-1 is true, statement-2 is false.
d)
Statement-1 is false, statement-2 is true.
|
|
T.ttttt answered |
When light of frequency f is incident on a metal surface that has a work function W, the maximum kinetic energy of the emitted electrons is given by:
KEmax=hf−ϕ
Note that this is the maximum possible kinetic energy because ϕ is the minimum energy necessary to liberate an electron.Increasing the frequency of the incident beam, keeping the number of incident photons fixed increases the maximum kinetic energy of the photoelectrons emitted.So, Statement-1 is False, Statement-2 is False.
KEmax=hf−ϕ
Note that this is the maximum possible kinetic energy because ϕ is the minimum energy necessary to liberate an electron.Increasing the frequency of the incident beam, keeping the number of incident photons fixed increases the maximum kinetic energy of the photoelectrons emitted.So, Statement-1 is False, Statement-2 is False.
The photoelectric threshold frequency of a metal is f0. When light of frequency 4f0is incident on the metal, the maximum K.E. of the emitted electron is- a)2 hf0
- b)4 hf0
- c)3 hf0
- d)hf0/ 2
Correct answer is option 'C'. Can you explain this answer?
The photoelectric threshold frequency of a metal is f0. When light of frequency 4f0is incident on the metal, the maximum K.E. of the emitted electron is
a)
2 hf0
b)
4 hf0
c)
3 hf0
d)
hf0/ 2
|
|
Hansa Sharma answered |
The maximum kinetic energy of the emitted electrons is given by
Kmax=hυ−ϕ0=h(4υ)−h(υ)=3hυ
Kmax=hυ−ϕ0=h(4υ)−h(υ)=3hυ
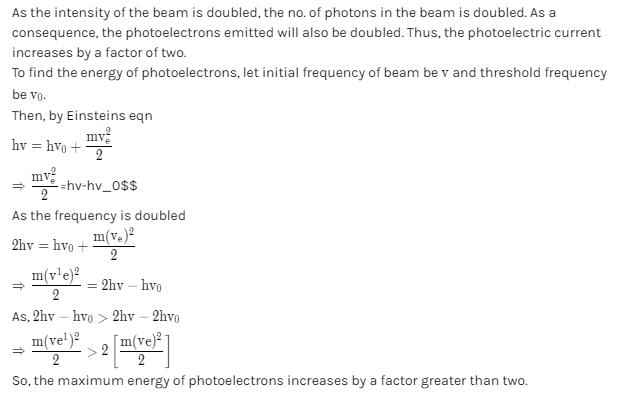
The frequency and the intensity of a beam of light falling on the surface of photoelectric material are increased by a factor of two (Treating efficiency of photoelectron generation as constant). This will :- a)Increase the maximum kinetic energy of the photoelectrons by a factor of greater than two and will have no effect on the magnitude of photoelectric current produced.
- b)Increase the maximum kinetic energy of the photo electrons and would increase the photoelectric current by a factor of two
- c)Increase the maximum energy of the photoelectrons by a factor greater than two, and photoelectric current by a factor of two
- d)Not produce any effect on the kinetic energy of the emitted electrons but will increase the photoelectric current by a factor of two
Correct answer is option 'C'. Can you explain this answer?
The frequency and the intensity of a beam of light falling on the surface of photoelectric material are increased by a factor of two (Treating efficiency of photoelectron generation as constant). This will :
a)
Increase the maximum kinetic energy of the photoelectrons by a factor of greater than two and will have no effect on the magnitude of photoelectric current produced.
b)
Increase the maximum kinetic energy of the photo electrons and would increase the photoelectric current by a factor of two
c)
Increase the maximum energy of the photoelectrons by a factor greater than two, and photoelectric current by a factor of two
d)
Not produce any effect on the kinetic energy of the emitted electrons but will increase the photoelectric current by a factor of two
|
|
Anu Sharma answered |
In photoelectric effect, stopping potential depends on- a)Frequency of the incident light
- b)Intensity of the incident light by varies source distance
- c)Emitter's properties
- d)Frequency and intensity of the incident light
Correct answer is option 'A,C'. Can you explain this answer?
In photoelectric effect, stopping potential depends on
a)
Frequency of the incident light
b)
Intensity of the incident light by varies source distance
c)
Emitter's properties
d)
Frequency and intensity of the incident light
|
|
Rutuja Ahuja answered |
Explanation:
The photoelectric effect is a phenomenon where electrons are emitted from the surface of a metal when light of a certain frequency or higher is incident on it. The stopping potential is the minimum potential required to stop the electrons emitted from the metal surface. The stopping potential depends on the frequency of the incident light and the emitter's properties.
Frequency of the incident light:
The stopping potential is directly proportional to the frequency of the incident light. This is because the energy of a photon is directly proportional to its frequency. The work function of the metal is the minimum energy required to remove an electron from its surface. If the energy of the incident photon is greater than the work function, then an electron will be emitted. The remaining energy of the photon will contribute to the kinetic energy of the emitted electron. Therefore, increasing the frequency of the incident light will increase the kinetic energy of the emitted electrons, and hence, the stopping potential required to stop them.
Emitter's properties:
The stopping potential also depends on the emitter's properties, such as its work function and the number of free electrons available for emission. The work function is the minimum energy required to remove an electron from the surface of the metal. Metals with a higher work function require more energy to remove an electron, and hence, a higher stopping potential to stop the emitted electrons. The number of free electrons available for emission also affects the stopping potential. Metals with a higher number of free electrons will require a higher stopping potential to stop the emitted electrons.
Intensity of the incident light:
The intensity of the incident light does not affect the stopping potential. This is because the number of emitted electrons is directly proportional to the intensity of the incident light. However, the kinetic energy of the emitted electrons will increase with increasing intensity, as more energy is transferred to each electron.
Frequency and intensity of the incident light:
The stopping potential depends only on the frequency of the incident light and the emitter's properties, not on the intensity of the incident light. Therefore, the correct answer is option A,C.
The photoelectric effect is a phenomenon where electrons are emitted from the surface of a metal when light of a certain frequency or higher is incident on it. The stopping potential is the minimum potential required to stop the electrons emitted from the metal surface. The stopping potential depends on the frequency of the incident light and the emitter's properties.
Frequency of the incident light:
The stopping potential is directly proportional to the frequency of the incident light. This is because the energy of a photon is directly proportional to its frequency. The work function of the metal is the minimum energy required to remove an electron from its surface. If the energy of the incident photon is greater than the work function, then an electron will be emitted. The remaining energy of the photon will contribute to the kinetic energy of the emitted electron. Therefore, increasing the frequency of the incident light will increase the kinetic energy of the emitted electrons, and hence, the stopping potential required to stop them.
Emitter's properties:
The stopping potential also depends on the emitter's properties, such as its work function and the number of free electrons available for emission. The work function is the minimum energy required to remove an electron from the surface of the metal. Metals with a higher work function require more energy to remove an electron, and hence, a higher stopping potential to stop the emitted electrons. The number of free electrons available for emission also affects the stopping potential. Metals with a higher number of free electrons will require a higher stopping potential to stop the emitted electrons.
Intensity of the incident light:
The intensity of the incident light does not affect the stopping potential. This is because the number of emitted electrons is directly proportional to the intensity of the incident light. However, the kinetic energy of the emitted electrons will increase with increasing intensity, as more energy is transferred to each electron.
Frequency and intensity of the incident light:
The stopping potential depends only on the frequency of the incident light and the emitter's properties, not on the intensity of the incident light. Therefore, the correct answer is option A,C.
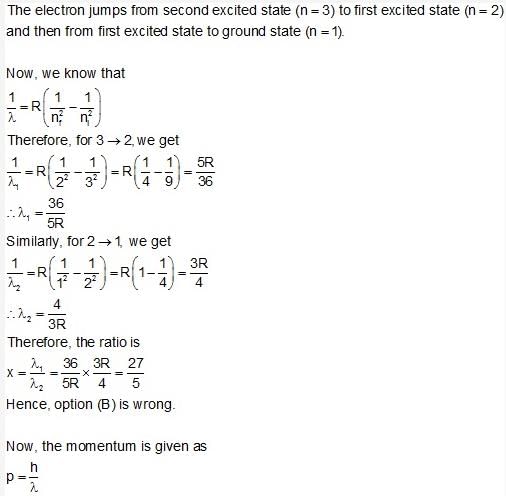
An electron in hydrogen atom first jumps from second excited state to first excited state and then, from first excited state to ground state. Let the ratio of wavelength, momentum and energy of photons in the two cases by x, y and z, then select the wrong answers :- a) z = 1/x
- b)x = 9/4
- c) y = 5/27
- d)z = 5/27
Correct answer is option 'B'. Can you explain this answer?
An electron in hydrogen atom first jumps from second excited state to first excited state and then, from first excited state to ground state. Let the ratio of wavelength, momentum and energy of photons in the two cases by x, y and z, then select the wrong answers :
a)
z = 1/x
b)
x = 9/4
c)
y = 5/27
d)
z = 5/27
|
|
Rajat Patel answered |

Light coming from a discharge tube filled with hydrogen falls on the cathode of the photoelectric cell. The work function of the surface of cathode is 4eV. Which one of the following values of the anode voltage (in Volts) with respect to the cathode will likely to make the photo current zero.- a)–4
- b)–6
- c)–8
- d)–10
Correct answer is option 'D'. Can you explain this answer?
Light coming from a discharge tube filled with hydrogen falls on the cathode of the photoelectric cell. The work function of the surface of cathode is 4eV. Which one of the following values of the anode voltage (in Volts) with respect to the cathode will likely to make the photo current zero.
a)
–4
b)
–6
c)
–8
d)
–10

|
Pragati Dey answered |
Energy of photon from H atom is gievn by the difference between the two energy level in which electron transit.for minimum wavelength, energy of the photon will be maximum.
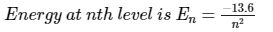
maximum energy will be

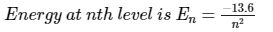
maximum energy will be

according to the einstein equation,Maximum kinetic energy if the emitted electron = energy of the incident radiation − work function
KE = 13.6 − 4 = 9.6 eV
hence potenial of the node must be greater than or equal to 9.6 volt with negatice polarity.
Hnece option (d) is correct.
Hnece option (d) is correct.
Statement-1: Two photons having equal wavelengths have equal linear momenta.
Statement-2: When light shows its photon character, each photon has a linear momentum p = .
- a) Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.
- b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.
- c)Statement-1 is true, statement-2 is false.
- d)Statement-1 is false, statement-2 is true.
Correct answer is option 'D'. Can you explain this answer?
Statement-1: Two photons having equal wavelengths have equal linear momenta.
Statement-2: When light shows its photon character, each photon has a linear momentum p = .
a)
Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.
b)
Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.
c)
Statement-1 is true, statement-2 is false.
d)
Statement-1 is false, statement-2 is true.
|
|
Aarav Khanna answered |
Correct Answer :- d
Explanation : Statement I is incorrect
The correct statement is : Two photons having Equal linear momenta have equal wavelengths.
In a photoelectric experiment, the potential difference V that must be maintained between the illuminated surface and the collector so as just to prevent any electron from reaching the collector is determined for different frequencies f of the incident illumination. The graph obtained is shown. The maximum kinetic energy of the electrons emitted at frequency f1 is 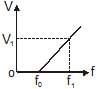
- a)hf1
- b).
- c)h(f1 – f0)
- d)eV1(f1 – f0)
Correct answer is option 'C'. Can you explain this answer?
In a photoelectric experiment, the potential difference V that must be maintained between the illuminated surface and the collector so as just to prevent any electron from reaching the collector is determined for different frequencies f of the incident illumination. The graph obtained is shown. The maximum kinetic energy of the electrons emitted at frequency f1 is
a)
hf1
b)
.
c)
h(f1 – f0)
d)
eV1(f1 – f0)
|
|
Shalini Choudhary answered |
A beam of ultraviolet light of all wavelengths passes through hydrogen gas at room temperature, in the x-direction. Assume that all photons emitted due to electron transition inside the gas emerge in the y-direction. Let A and B denote the lights emerging from the gas in the x and y directions respectively.- a)Some of the incident wavelengths will be absent in A
- b)Only those wavelengths will be present in B which are absent in A
- c)B will contain some visible light.
- d)B will contain some infrared light.
Correct answer is option 'A,C,D'. Can you explain this answer?
A beam of ultraviolet light of all wavelengths passes through hydrogen gas at room temperature, in the x-direction. Assume that all photons emitted due to electron transition inside the gas emerge in the y-direction. Let A and B denote the lights emerging from the gas in the x and y directions respectively.
a)
Some of the incident wavelengths will be absent in A
b)
Only those wavelengths will be present in B which are absent in A
c)
B will contain some visible light.
d)
B will contain some infrared light.
|
|
Harsh Desai answered |
Given, incident wavelengths travel in x-direction which are in the U.V region, which means they can excite an electron from n=1 to higher levels. Hence, some of the incident wavelengths will be absent in A.
Photons emerging due to transitions of electrons to their ground state contains visible region wavelengths if transition is done into n=2. If transition is done into n>2, photons contain I.R wavelengths.
Therefore, A,C,D is the correct choice.
Photons emerging due to transitions of electrons to their ground state contains visible region wavelengths if transition is done into n=2. If transition is done into n>2, photons contain I.R wavelengths.
Therefore, A,C,D is the correct choice.
An isolated metal body is illuminated with monochromatic light and is observed to become charged to a steady positive potential 1.0 V with respect to the surrounding. The work function of the metal is 3.0 eV. The frequency of the incident light is ___________________ eV.
Correct answer is '4.0'. Can you explain this answer?
An isolated metal body is illuminated with monochromatic light and is observed to become charged to a steady positive potential 1.0 V with respect to the surrounding. The work function of the metal is 3.0 eV. The frequency of the incident light is ___________________ eV.
|
|
Mihir Yadav answered |
By Einstein's Photoelectric equation
eV=hν−ϕ
where V=1V= cut off voltage
⟹ν= (eV+ϕ)/ h =4eV/h=4×1.6×10−19/6.63×10−34
⟹ν=9.6×1014Hz
eV=hν−ϕ
where V=1V= cut off voltage
⟹ν= (eV+ϕ)/ h =4eV/h=4×1.6×10−19/6.63×10−34
⟹ν=9.6×1014Hz
Statement-1: Work function of aluminum is 4.2 eV. If two photons each of energy 2.5 eV strikes on a piece of aluminum, the photo electric emission does not occur.Statement-2: In photo electric effect a single photon interacts with a single electron and electron is emitted only if energy of each incident photon is greater then the work function.- a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.
- b) Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.
- c)Statement-1 is true, statement-2 is false.
- d)Statement-1 is false, statement-2 is true.
Correct answer is option 'A'. Can you explain this answer?
Statement-1: Work function of aluminum is 4.2 eV. If two photons each of energy 2.5 eV strikes on a piece of aluminum, the photo electric emission does not occur.
Statement-2: In photo electric effect a single photon interacts with a single electron and electron is emitted only if energy of each incident photon is greater then the work function.
a)
Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.
b)
Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.
c)
Statement-1 is true, statement-2 is false.
d)
Statement-1 is false, statement-2 is true.
|
|
Nishtha Dasgupta answered |
Statement-1: Work function of aluminum is 4.2 eV. If two photons each of energy 2.5 eV strikes on a piece of aluminum, the photoelectric emission does not occur.
Statement-2: In the photoelectric effect, a single photon interacts with a single electron, and the electron is emitted only if the energy of each incident photon is greater than the work function.
To analyze the given statements, let's break them down and discuss each one separately.
Statement-1: Work function of aluminum is 4.2 eV. If two photons each of energy 2.5 eV strikes on a piece of aluminum, the photoelectric emission does not occur.
The work function of a material is the minimum amount of energy required to remove an electron from the material's surface. In this case, the work function of aluminum is stated as 4.2 eV. When two photons, each with an energy of 2.5 eV, strike the aluminum surface, the total energy of the photons is 5 eV (2.5 eV + 2.5 eV).
Statement-2: In the photoelectric effect, a single photon interacts with a single electron, and the electron is emitted only if the energy of each incident photon is greater than the work function.
The photoelectric effect is the phenomenon where electrons are emitted from a material's surface when it is exposed to light. According to the statement, a single photon interacts with a single electron, and the electron is emitted only if the energy of the incident photon is greater than the work function of the material.
Explanation:
Both statements are true, and statement-2 provides a correct explanation for statement-1. Here's why:
When the two photons with energies of 2.5 eV each strike the aluminum surface, their total energy is 5 eV (2.5 eV + 2.5 eV). In the photoelectric effect, the energy of each incident photon should be greater than the work function for electron emission to occur.
In this case, the energy of each incident photon (2.5 eV) is less than the work function of aluminum (4.2 eV). Therefore, even though the total energy of the two photons is sufficient (5 eV), the energy of each individual photon is not enough to overcome the work function barrier. As a result, the photoelectric emission does not occur.
Thus, statement-2 correctly explains why the photoelectric emission does not occur in this scenario, supporting statement-1.
In conclusion, both statements are true, and statement-2 provides a correct explanation for statement-1.
Statement-2: In the photoelectric effect, a single photon interacts with a single electron, and the electron is emitted only if the energy of each incident photon is greater than the work function.
To analyze the given statements, let's break them down and discuss each one separately.
Statement-1: Work function of aluminum is 4.2 eV. If two photons each of energy 2.5 eV strikes on a piece of aluminum, the photoelectric emission does not occur.
The work function of a material is the minimum amount of energy required to remove an electron from the material's surface. In this case, the work function of aluminum is stated as 4.2 eV. When two photons, each with an energy of 2.5 eV, strike the aluminum surface, the total energy of the photons is 5 eV (2.5 eV + 2.5 eV).
Statement-2: In the photoelectric effect, a single photon interacts with a single electron, and the electron is emitted only if the energy of each incident photon is greater than the work function.
The photoelectric effect is the phenomenon where electrons are emitted from a material's surface when it is exposed to light. According to the statement, a single photon interacts with a single electron, and the electron is emitted only if the energy of the incident photon is greater than the work function of the material.
Explanation:
Both statements are true, and statement-2 provides a correct explanation for statement-1. Here's why:
When the two photons with energies of 2.5 eV each strike the aluminum surface, their total energy is 5 eV (2.5 eV + 2.5 eV). In the photoelectric effect, the energy of each incident photon should be greater than the work function for electron emission to occur.
In this case, the energy of each incident photon (2.5 eV) is less than the work function of aluminum (4.2 eV). Therefore, even though the total energy of the two photons is sufficient (5 eV), the energy of each individual photon is not enough to overcome the work function barrier. As a result, the photoelectric emission does not occur.
Thus, statement-2 correctly explains why the photoelectric emission does not occur in this scenario, supporting statement-1.
In conclusion, both statements are true, and statement-2 provides a correct explanation for statement-1.
If radiation of allow wavelengths from ultraviolet to infrared is passed through hydrogen agas at room temperature, absorption lines will be observed in the :- a)Lyman series
- b) Balmer series
- c)Both (A) and (B)
- d)Neither (A) nor (B)
Correct answer is option 'A'. Can you explain this answer?
If radiation of allow wavelengths from ultraviolet to infrared is passed through hydrogen agas at room temperature, absorption lines will be observed in the :
a)
Lyman series
b)
Balmer series
c)
Both (A) and (B)
d)
Neither (A) nor (B)

|
Shilpa Saha answered |
At room temperature, nearly all the atoms in hydrogen gas will be in the ground state. When light passes through the gas, photons are absorbed, causing electrons to make transitions to higher states and creating absorption lines. These lines correspond to the Lyman series since that is the series of transitions involving the ground state or n = 1 level. Since there are virtually no atoms in higher energy states, photons corresponding to transitions from n > 2 to higher states will not be absorbed.
In the hydrogen atom, if the reference level of potential energy is assumed to be zero at the ground state level. Choose the incorrect statement.- a)The total energy of the shell increases with increase in the value of n
- b)The total energy of the shell decrease with increase in the value of n.
- c)The difference in total energy of any two shells remains the same.
- d)The total energy at the ground state becomes 13.6 eV.
Correct answer is option 'A'. Can you explain this answer?
In the hydrogen atom, if the reference level of potential energy is assumed to be zero at the ground state level. Choose the incorrect statement.
a)
The total energy of the shell increases with increase in the value of n
b)
The total energy of the shell decrease with increase in the value of n.
c)
The difference in total energy of any two shells remains the same.
d)
The total energy at the ground state becomes 13.6 eV.
|
|
Srestha Chopra answered |
Correct Answer :- a
Explanation : When the electron changes levels, it decreases energy and the atom emits photons. The photon is emitted with the electron moving from a higher energy level to a lower energy level. The energy of the photon is the exact energy that is lost by the electron moving to its lower energy level.
Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l = 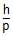 where, p is the linear momentum and both have same K.E.
where, p is the linear momentum and both have same K.E.- a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.
- b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.
- c)Statement-1 is true, statement-2 is false.
- d)Statement-1 is false, statement-2 is true.
Correct answer is option 'A'. Can you explain this answer?
Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.
Statement-2: De-Broglie wavelength associated with a moving particle is l = 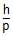 where, p is the linear momentum and both have same K.E.
where, p is the linear momentum and both have same K.E.
a)
Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.
b)
Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.
c)
Statement-1 is true, statement-2 is false.
d)
Statement-1 is false, statement-2 is true.
|
|
Shubham Jain answered |
de Broglie Wavelength for Electrons
In the case of electrons going in circles around the nuclei in atoms, the de Broglie waves exist as a closed-loop, such that they can exist only as standing waves, and fit evenly around the loop. Because of this requirement, the electrons in atoms circle the nucleus in particular configurations, or states, which are called stationary orbits.
de Broglie Wavelength Formula and Derivation
de Broglie reasoned that matter also can show wave-particle duality, just like light, since light can behave both as a wave (it can be diffracted and it has a wavelength) and as a particle (it contains packets of energy hν). And also reasoned that matter would follow the same equation for wavelength as light namely,
λ = h / p
Where p is the linear momentum, as shown by Einstein.
Derivation
de Broglie derived the above relationship as follows:
1) E = hν for a photon and λν = c for an electromagnetic wave.
2) E = mc2, means λ = h/mc, which is equivalent to λ = h/p.
Note: m is the relativistic mass, and not the rest mass; since the rest mass of a photon is zero.
Now, if a particle is moving with a velocity v, the momentum p = mv and hence λ = h / mv
Therefore, the de Broglie wavelength formula is expressed as;
λ = h / mv
In the case of electrons going in circles around the nuclei in atoms, the de Broglie waves exist as a closed-loop, such that they can exist only as standing waves, and fit evenly around the loop. Because of this requirement, the electrons in atoms circle the nucleus in particular configurations, or states, which are called stationary orbits.
de Broglie Wavelength Formula and Derivation
de Broglie reasoned that matter also can show wave-particle duality, just like light, since light can behave both as a wave (it can be diffracted and it has a wavelength) and as a particle (it contains packets of energy hν). And also reasoned that matter would follow the same equation for wavelength as light namely,
λ = h / p
Where p is the linear momentum, as shown by Einstein.
Derivation
de Broglie derived the above relationship as follows:
1) E = hν for a photon and λν = c for an electromagnetic wave.
2) E = mc2, means λ = h/mc, which is equivalent to λ = h/p.
Note: m is the relativistic mass, and not the rest mass; since the rest mass of a photon is zero.
Now, if a particle is moving with a velocity v, the momentum p = mv and hence λ = h / mv
Therefore, the de Broglie wavelength formula is expressed as;
λ = h / mv
Chapter doubts & questions for Photoelectric Effect - Physics for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Photoelectric Effect - Physics for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Physics for EmSAT Achieve
208 videos|329 docs|212 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily










