All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Ionic Compounds for EmSAT Achieve Exam
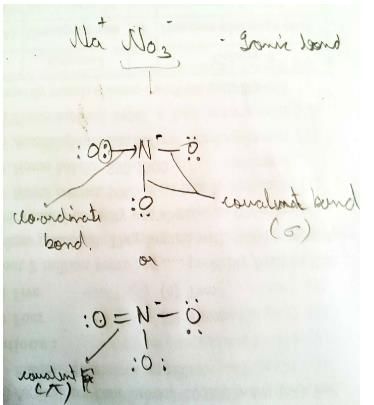
Different kinds of bonds and interaction present within CuSO4 • 5H2O. They can beI. σ-bond
II. π-bond
III. coordinate bond
IV. electrostatic force of attraction
V. H-bond due to dipole-dipole interaction
VI. H-bond due to ion-dipole interaction Select the correct types of bonds/interactions.- a)I, II and III
- b)I, III and IV
- c)I, III, V and VI
- d)All of these
Correct answer is option 'D'. Can you explain this answer?
Different kinds of bonds and interaction present within CuSO4 • 5H2O. They can be
I. σ-bond
II. π-bond
III. coordinate bond
IV. electrostatic force of attraction
V. H-bond due to dipole-dipole interaction
VI. H-bond due to ion-dipole interaction
II. π-bond
III. coordinate bond
IV. electrostatic force of attraction
V. H-bond due to dipole-dipole interaction
VI. H-bond due to ion-dipole interaction
Select the correct types of bonds/interactions.
a)
I, II and III
b)
I, III and IV
c)
I, III, V and VI
d)
All of these
|
|
Krishna Iyer answered |
IV. Electrostatic force of attraction between
V. H2O molecules joined together by dipole-dipole interaction.
VI. Outer H2O molecule joined to
Covalent nature of NaF, Na2O and Na3N in the increasing order is- a)Na3N < Na2O < NaF
- b)NaF < Na2O < Na3N
- c)Na2O < NaF < Na3N
- d)Na2O < Na3 N < NaF
Correct answer is option 'B'. Can you explain this answer?
Covalent nature of NaF, Na2O and Na3N in the increasing order is
a)
Na3N < Na2O < NaF
b)
NaF < Na2O < Na3N
c)
Na2O < NaF < Na3N
d)
Na2O < Na3 N < NaF

|
Athul Patel answered |
By Fajans' rule,
Smaller the size of cation, larger the size of anion. Larger the charge on cation or anion, larger the polarising power and thus, greater the covalent nature.
NaF, Na2O, Na3N, Na+ is same.
Smaller the size of cation, larger the size of anion. Larger the charge on cation or anion, larger the polarising power and thus, greater the covalent nature.
NaF, Na2O, Na3N, Na+ is same.
Can you explain the answer of this question below:Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The correct statement for the molecule Csl-3 is
- A:
it is a covalent compound
- B:
it contains Cs3+ and l- ions
- C:
it contains Cs+ and l-3 ions
- D:
it contains cs+ , l- ions and lattice l2 molecule
The answer is c.
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The correct statement for the molecule Csl-3 is
it is a covalent compound
it contains Cs3+ and l- ions
it contains Cs+ and l-3 ions
it contains cs+ , l- ions and lattice l2 molecule
|
|
Gaurav Kumar answered |
Cs is electropositive element hence, it forms Cs+ ion. Hence, no covalent bonding.
Cs+ has stable inert gas configuration thus, Cs3+ is not formed.
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. The correct statement for the molecule Csl-3 is- a)it is a covalent compound
- b)it contains Cs3+ and l- ions
- c)it contains Cs+ and l-3 ions
- d)it contains cs+ , l- ions and lattice l2 molecule
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The correct statement for the molecule Csl-3 is
a)
it is a covalent compound
b)
it contains Cs3+ and l- ions
c)
it contains Cs+ and l-3 ions
d)
it contains cs+ , l- ions and lattice l2 molecule

|
Devansh Goyal answered |
Cs is electropositive element hence, it forms Cs+ ion. Hence, no covalent bonding.
Cs+ has stable inert gas configuration thus, Cs3+ is not formed.
Aqueous solution of a mixture contains LiCI, CuCI, NaCI and AICI3. This is shaken with ether. What is/are left in water?- a)LiCI, NaCI
- b)NaCI, AICI3
- c)Only CuCI
- d)Only NaCI
Correct answer is option 'D'. Can you explain this answer?
Aqueous solution of a mixture contains LiCI, CuCI, NaCI and AICI3. This is shaken with ether. What is/are left in water?
a)
LiCI, NaCI
b)
NaCI, AICI3
c)
Only CuCI
d)
Only NaCI

|
Mohit Patel answered |
Polarising power of Li+ > Na+ and AI3+ > Na+
Thus, LiCI and AICI3 have covalent nature and extracted into ether.
CuCI due to d-electrons has covalent nature. (Polarising power of Cu+ > Na+).
Thus, only NaCI (ionic) remains in water.
Thus, LiCI and AICI3 have covalent nature and extracted into ether.
CuCI due to d-electrons has covalent nature. (Polarising power of Cu+ > Na+).
Thus, only NaCI (ionic) remains in water.
Out of the following, maximum covalent nature is in- a)NaF
- b)MgO
- c)AIN
- d)SiC
Correct answer is option 'D'. Can you explain this answer?
Out of the following, maximum covalent nature is in
a)
NaF
b)
MgO
c)
AIN
d)
SiC

|
Nilesh Goyal answered |
By Fajans rule,
Smaller the size of cation, larger the size of anion. Larger the charge on cation or anion, larger the polarising power and thus, greater the covalent nature.

Smaller the size of cation, larger the size of anion. Larger the charge on cation or anion, larger the polarising power and thus, greater the covalent nature.
Which pair is not in the correct order of lattice energy?- a)KCl < MgO
- b)AIN > MgO
- c)BeCO3 > MgCO3
- d)BeCO3 = MgCO3
Correct answer is option 'D'. Can you explain this answer?
Which pair is not in the correct order of lattice energy?
a)
KCl < MgO
b)
AIN > MgO
c)
BeCO3 > MgCO3
d)
BeCO3 = MgCO3
|
|
Arka Bose answered |
Explanation:
Lattice Energy:
Lattice energy is the energy released when gaseous ions form a solid compound. It is a measure of the strength of the ionic bond in a compound.
Correct Order of Lattice Energy:
The correct order of lattice energy is determined by the charges of the ions and the size of the ions. Generally, the higher the charges of the ions and the smaller their size, the higher the lattice energy.
Incorrect Pair:
The pair that is not in the correct order of lattice energy is option D - BeCO3 = MgCO3. This is because the lattice energy of a compound is influenced by the charges of the ions and the sizes of the ions. In this case, the charges of the ions in BeCO3 and MgCO3 are the same (Be2+ and Mg2+), but the size of the ions is different. Beryllium ions (Be2+) are smaller in size compared to magnesium ions (Mg2+), which results in a higher lattice energy for BeCO3 compared to MgCO3. Therefore, the correct order should be BeCO3 > MgCO3.
Correct Order of Lattice Energy:
- KCl > MgO
- AlN > MgO
- BeCO3 > MgCO3
Therefore, the correct order of lattice energy among the given pairs is:
a) KCl > MgO
b) AlN > MgO
c) BeCO3 > MgCO3
Lattice Energy:
Lattice energy is the energy released when gaseous ions form a solid compound. It is a measure of the strength of the ionic bond in a compound.
Correct Order of Lattice Energy:
The correct order of lattice energy is determined by the charges of the ions and the size of the ions. Generally, the higher the charges of the ions and the smaller their size, the higher the lattice energy.
Incorrect Pair:
The pair that is not in the correct order of lattice energy is option D - BeCO3 = MgCO3. This is because the lattice energy of a compound is influenced by the charges of the ions and the sizes of the ions. In this case, the charges of the ions in BeCO3 and MgCO3 are the same (Be2+ and Mg2+), but the size of the ions is different. Beryllium ions (Be2+) are smaller in size compared to magnesium ions (Mg2+), which results in a higher lattice energy for BeCO3 compared to MgCO3. Therefore, the correct order should be BeCO3 > MgCO3.
Correct Order of Lattice Energy:
- KCl > MgO
- AlN > MgO
- BeCO3 > MgCO3
Therefore, the correct order of lattice energy among the given pairs is:
a) KCl > MgO
b) AlN > MgO
c) BeCO3 > MgCO3
Mg2+, 02- is formed when- a)ionisation energy of Mg is low
- b)electron affinity of O is low
- c)ionisation energy of O is low
- d)ionisation energy of Mg is high
Correct answer is option 'A'. Can you explain this answer?
Mg2+, 02- is formed when
a)
ionisation energy of Mg is low
b)
electron affinity of O is low
c)
ionisation energy of O is low
d)
ionisation energy of Mg is high
|
|
Arka Bose answered |
Explanation:
Ionization Energy:
Ionization energy is the energy required to remove an electron from an atom. The lower the ionization energy, the easier it is to remove an electron.
Electron Affinity:
Electron affinity is the energy released when an electron is added to an atom. The lower the electron affinity, the less likely an atom is to gain an electron.
Formation of Mg2+, O2-:
- In the case of Mg2+, the ionization energy of Mg is relatively low, making it easier for magnesium to lose two electrons and form the Mg2+ ion.
- In the case of O2-, the electron affinity of oxygen is relatively low, meaning it is less likely for oxygen to gain two electrons and form the O2- ion.
Therefore, Mg2+ and O2- are formed because of the low ionization energy of Mg, allowing it to lose electrons easily, while the low electron affinity of O prevents it from gaining electrons easily.
Ionization Energy:
Ionization energy is the energy required to remove an electron from an atom. The lower the ionization energy, the easier it is to remove an electron.
Electron Affinity:
Electron affinity is the energy released when an electron is added to an atom. The lower the electron affinity, the less likely an atom is to gain an electron.
Formation of Mg2+, O2-:
- In the case of Mg2+, the ionization energy of Mg is relatively low, making it easier for magnesium to lose two electrons and form the Mg2+ ion.
- In the case of O2-, the electron affinity of oxygen is relatively low, meaning it is less likely for oxygen to gain two electrons and form the O2- ion.
Therefore, Mg2+ and O2- are formed because of the low ionization energy of Mg, allowing it to lose electrons easily, while the low electron affinity of O prevents it from gaining electrons easily.
Select the correct statement(s).- a)CuCI is covalent and NaCI is ionic
- b)Inner electrons have poor shielding effect on the nucleus and thus, electronegativity of the 18-electrons shell is increased
- c)Cations with 18-electrons shell have greater polarising power than the cations with 8-electrons shell
- d)All of the above are correct statements
Correct answer is option 'D'. Can you explain this answer?
Select the correct statement(s).
a)
CuCI is covalent and NaCI is ionic
b)
Inner electrons have poor shielding effect on the nucleus and thus, electronegativity of the 18-electrons shell is increased
c)
Cations with 18-electrons shell have greater polarising power than the cations with 8-electrons shell
d)
All of the above are correct statements

|
Sagar Mukherjee answered |
CuCl is covalent and NaCl is ionic
--------------------------------------
The correct statement is (a) CuCl is covalent and NaCl is ionic.
Explanation:
CuCl is covalent because copper (Cu) is a transition metal and chlorine (Cl) is a nonmetal. Transition metals tend to form covalent compounds because they have multiple oxidation states and can share electrons with nonmetals. In CuCl, the copper atom shares one electron with the chlorine atom to form a covalent bond.
On the other hand, NaCl is ionic because sodium (Na) is a metal and chlorine (Cl) is a nonmetal. Metals tend to lose electrons to form cations, while nonmetals tend to gain electrons to form anions. In NaCl, sodium loses one electron to chlorine, resulting in the formation of Na+ cation and Cl- anion. The electrostatic attraction between the oppositely charged ions leads to the formation of an ionic bond.
Inner electrons have poor shielding effect on the nucleus and thus, electronegativity of the 18-electrons shell is increased
-------------------------------------------------------------------------------------------------------------------------
The statement (b) "Inner electrons have poor shielding effect on the nucleus and thus, electronegativity of the 18-electrons shell is increased" is incorrect.
Explanation:
Inner electrons do not have a poor shielding effect on the nucleus. In fact, inner electrons provide better shielding to the outer electrons from the positively charged nucleus. This is because inner electrons are closer to the nucleus and their negative charge repels the outer electrons, reducing the attractive force between the outer electrons and the nucleus. As a result, the electronegativity of the 18-electron shell is decreased, not increased. Electronegativity generally increases across a period from left to right, as the effective nuclear charge increases, but it decreases down a group due to increased shielding by inner electron shells.
Cations with 18-electron shells have greater polarising power than the cations with 8-electron shells
----------------------------------------------------------------------------------------------------------------
The statement (c) "Cations with 18-electron shells have greater polarising power than the cations with 8-electron shells" is incorrect.
Explanation:
Cations with 8-electron shells have greater polarising power than cations with 18-electron shells. Polarising power refers to the ability of a cation to distort the electron cloud of an anion in an ionic compound. Cations with smaller size and higher charge have greater polarising power because they can more effectively pull the electrons of the anion towards themselves.
Cations with 8-electron shells, such as Al3+ or Fe3+, have high charge density and smaller size compared to cations with 18-electron shells, such as Na+ or K+. Therefore, cations with 8-electron shells have greater polarising power as they can distort the electron cloud of the anion more effectively, leading to stronger ionic bonds.
--------------------------------------
The correct statement is (a) CuCl is covalent and NaCl is ionic.
Explanation:
CuCl is covalent because copper (Cu) is a transition metal and chlorine (Cl) is a nonmetal. Transition metals tend to form covalent compounds because they have multiple oxidation states and can share electrons with nonmetals. In CuCl, the copper atom shares one electron with the chlorine atom to form a covalent bond.
On the other hand, NaCl is ionic because sodium (Na) is a metal and chlorine (Cl) is a nonmetal. Metals tend to lose electrons to form cations, while nonmetals tend to gain electrons to form anions. In NaCl, sodium loses one electron to chlorine, resulting in the formation of Na+ cation and Cl- anion. The electrostatic attraction between the oppositely charged ions leads to the formation of an ionic bond.
Inner electrons have poor shielding effect on the nucleus and thus, electronegativity of the 18-electrons shell is increased
-------------------------------------------------------------------------------------------------------------------------
The statement (b) "Inner electrons have poor shielding effect on the nucleus and thus, electronegativity of the 18-electrons shell is increased" is incorrect.
Explanation:
Inner electrons do not have a poor shielding effect on the nucleus. In fact, inner electrons provide better shielding to the outer electrons from the positively charged nucleus. This is because inner electrons are closer to the nucleus and their negative charge repels the outer electrons, reducing the attractive force between the outer electrons and the nucleus. As a result, the electronegativity of the 18-electron shell is decreased, not increased. Electronegativity generally increases across a period from left to right, as the effective nuclear charge increases, but it decreases down a group due to increased shielding by inner electron shells.
Cations with 18-electron shells have greater polarising power than the cations with 8-electron shells
----------------------------------------------------------------------------------------------------------------
The statement (c) "Cations with 18-electron shells have greater polarising power than the cations with 8-electron shells" is incorrect.
Explanation:
Cations with 8-electron shells have greater polarising power than cations with 18-electron shells. Polarising power refers to the ability of a cation to distort the electron cloud of an anion in an ionic compound. Cations with smaller size and higher charge have greater polarising power because they can more effectively pull the electrons of the anion towards themselves.
Cations with 8-electron shells, such as Al3+ or Fe3+, have high charge density and smaller size compared to cations with 18-electron shells, such as Na+ or K+. Therefore, cations with 8-electron shells have greater polarising power as they can distort the electron cloud of the anion more effectively, leading to stronger ionic bonds.
Direction (Q. Nos. 21-22) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)A+B- (ionic) is formed in the following steps from gaseous atoms
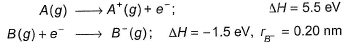
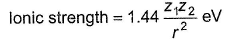 Q.
Q.
How much energy is required to transfer one electron from A to B? - a)4.0 eV
- b)-4.0 eV
- c)7.0 eV
- d)- 7.0 eV
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 21-22) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
A+B- (ionic) is formed in the following steps from gaseous atoms
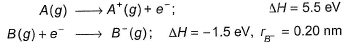
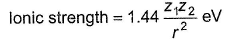
Q.
How much energy is required to transfer one electron from A to B?
How much energy is required to transfer one electron from A to B?
a)
4.0 eV
b)
-4.0 eV
c)
7.0 eV
d)
- 7.0 eV

|
Uday Chakraborty answered |
Solubility of CuS(l), ZnS (II) and Na2S (III) in water is in the order- a)I < II < III
- b)III < l < II
- c)II < I < Ill
- d)Ill < II < I
Correct answer is option 'A'. Can you explain this answer?
Solubility of CuS(l), ZnS (II) and Na2S (III) in water is in the order
a)
I < II < III
b)
III < l < II
c)
II < I < Ill
d)
Ill < II < I

|
Aravind Nambiar answered |
By Fajans' rule,
Smaller the size of cation, larger the size of anion.
Larger the charge ord-electrons present, then larger the polarising power of cation and larger the covalent nature and smaller the solubility in water.
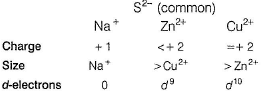
Cu2+ due to shielding effect causes more screening than Zn2+.
Thus, polarising power of Na+ <Zn2+ < Cu2+ hence, ionic nature and solubility in water is in order
CuS < ZnS < Na2S
Smaller the size of cation, larger the size of anion.
Larger the charge ord-electrons present, then larger the polarising power of cation and larger the covalent nature and smaller the solubility in water.
Cu2+ due to shielding effect causes more screening than Zn2+.
Thus, polarising power of Na+ <Zn2+ < Cu2+ hence, ionic nature and solubility in water is in order
CuS < ZnS < Na2S
Arrange NaCI, MgCI2, AICI3, SiCI4 in increasing solubility in ether (non-polar solvent).- a)NaCI < MgCI2 < AlCI3 < SiCI4
- b)SiCI4 < AICI3 < MgCl2 < NaCI
- c)SiCI2 = AICI3 < MgCI2 < NaCI
- d)Can’t be decided
Correct answer is option 'A'. Can you explain this answer?
Arrange NaCI, MgCI2, AICI3, SiCI4 in increasing solubility in ether (non-polar solvent).
a)
NaCI < MgCI2 < AlCI3 < SiCI4
b)
SiCI4 < AICI3 < MgCl2 < NaCI
c)
SiCI2 = AICI3 < MgCI2 < NaCI
d)
Can’t be decided

|
Anand Khanna answered |
Like dissolves like. Polar solvents (as H2O) dissolve polar compounds (ionic compounds) and non-polar solvents (as ether) dissolve non-polar compounds (covalent compounds).
By Fajans’ rule smaller the size of cation, larger the size of anion. Larger the charge on cation or anion then larger the polarising power and thus, larger the covalent nature and thus, solubility in non-polar solvents.
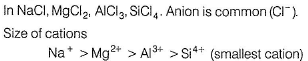
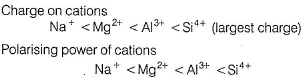
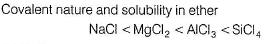
By Fajans’ rule smaller the size of cation, larger the size of anion. Larger the charge on cation or anion then larger the polarising power and thus, larger the covalent nature and thus, solubility in non-polar solvents.
Direction (Q. Nos. 23) This section contains 1 question. when worked out will result in an integerfrom to 9 (both inclusive)Q.
An ionic bond is established between a positive ion A+ and negative ion B- . How many times strength of the ionic bond is affected by doubling the charge on A+ and making the radius halved?
Correct answer is '8'. Can you explain this answer?
Direction (Q. Nos. 23) This section contains 1 question. when worked out will result in an integerfrom to 9 (both inclusive)
Q.
An ionic bond is established between a positive ion A+ and negative ion B- . How many times strength of the ionic bond is affected by doubling the charge on A+ and making the radius halved?
An ionic bond is established between a positive ion A+ and negative ion B- . How many times strength of the ionic bond is affected by doubling the charge on A+ and making the radius halved?

|
Ameya Mukherjee answered |
Thus, ionic strength becomes 8 times.
Lewis structure of the elements M and Z are shown below.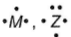 Compound formed is
Compound formed is- a)M5Z3
- b)M2Z3
- c)MZ
- d)MZ3
Correct answer is option 'C'. Can you explain this answer?
Lewis structure of the elements M and Z are shown below.
Compound formed is
a)
M5Z3
b)
M2Z3
c)
MZ
d)
MZ3

|
Raghav Chakraborty answered |
(Three electrons extra than octet, it can donate three electrons)
(Three electrons short of octet, it can gain three electrons)
Thus, M3+ Z3-
Compound is MZ,
Lewis structure of MgCI2 and Al2O3 are- a)
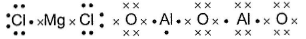
- b)
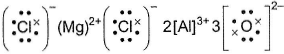
- c)Both (a) and (b) are correct
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Lewis structure of MgCI2 and Al2O3 are
a)
b)
c)
Both (a) and (b) are correct
d)
None of the above
|
|
Anmol Chauhan answered |
Mg(1s2 2s2 2p63s2) is an electropositive element and Cl(1s2 2s2 2p6 3s2 3p5)is an electronegative element.
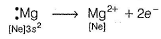
Mg2+ attains stable inert gas configuration.
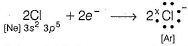
Cl- also attains stable inert gas configuration.
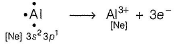
Al3+ attains stable inert gas configuration.
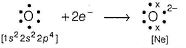
O2- attains stable inert gas configuration.
Mg2+ attains stable inert gas configuration.
Cl- also attains stable inert gas configuration.
Al3+ attains stable inert gas configuration.
O2- attains stable inert gas configuration.
An ionic compound A+B- is most like to be formed from A and B when - a)ionisation energy of A is low
- b)electron affinity of B is high
- c)electron affinity of A is high
- d)ionisation energy of A is high
Correct answer is option 'A,B'. Can you explain this answer?
An ionic compound A+B- is most like to be formed from A and B when
a)
ionisation energy of A is low
b)
electron affinity of B is high
c)
electron affinity of A is high
d)
ionisation energy of A is high

|
Gowri Chavan answered |
A → A+ + e- is favourable since, IE of A is low.
B + e- → B- is favourable since, EA of B is high.
Thus, A+B- is favoured by low IE of A and high EA of B.
B + e- → B- is favourable since, EA of B is high.
Thus, A+B- is favoured by low IE of A and high EA of B.
Which of the following Lewis acid-Lewis base interactions are associated with the expansion of octet?- a)
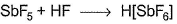
- b)
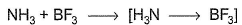
- c)
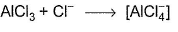
- d)
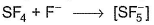
Correct answer is option 'A,D'. Can you explain this answer?
Which of the following Lewis acid-Lewis base interactions are associated with the expansion of octet?
a)
b)
c)
d)

|
Akash Shah answered |
(a) In SbF5 (expansion of octet from 5 to 10 and further to 12).
(b) NH3, BF3 both have complete octet. NH3 is electron rich, donates lone pair to BF3.
(c) AICI3 is electron deficient, accepts lone pair from Cl-.
(d) In SF4, expansion of octet from 8 to 10 and further to 12.
(b) NH3, BF3 both have complete octet. NH3 is electron rich, donates lone pair to BF3.
(c) AICI3 is electron deficient, accepts lone pair from Cl-.
(d) In SF4, expansion of octet from 8 to 10 and further to 12.
Out of the following ions, which pair wil make the compound most covalent?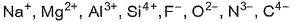
- a)Na+, F-
- b)Mg2+, O2-
- c)Al3+, N3-
- d)Si4+,C4-
Correct answer is option 'D'. Can you explain this answer?
Out of the following ions, which pair wil make the compound most covalent?
a)
Na+, F-
b)
Mg2+, O2-
c)
Al3+, N3-
d)
Si4+,C4-

|
Milan Roy answered |
By Fajans’ rule, smaller the size of cation, larger the size of anion, larger the charge on cation or anion,
d- and f-electrons present on the ion then greater the polarising power of the ion to polarise the other ion, thus larger the covalent nature.
d- and f-electrons present on the ion then greater the polarising power of the ion to polarise the other ion, thus larger the covalent nature.
Thus, com pound with Si4+ (smallest cation) and C4- (largest anion) will be most covalent.
Most stable compound is- a)LiF
- b)LiCI
- c)LiBr
- d)Lil
Correct answer is option 'A'. Can you explain this answer?
Most stable compound is
a)
LiF
b)
LiCI
c)
LiBr
d)
Lil

|
Saikat Dasgupta answered |
By Fajans’ rule,
Smaller the size of cation, larger the size of anion, larger the charge, then larger the polarising power, larger the covalent nature, means low melting point.
Smaller the ionic nature, means low melting point
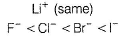

Hence, most stable is LiF.
Smaller the size of cation, larger the size of anion, larger the charge, then larger the polarising power, larger the covalent nature, means low melting point.
Smaller the ionic nature, means low melting point
Hence, most stable is LiF.
Direction (Q. Nos. 17-20) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.Q. Which of the following can be calculated based on Born-Haber cycle of formation of a lattice A+B- (s) from A(s) and 6 (g) ?- a)Lattice energy of A+B- (s)
- b)Electron affinity of B (g)
- c)Hydration energy of A+(g)
- d)Ionisation energy of A (s).
Correct answer is option 'A,B,D'. Can you explain this answer?
Direction (Q. Nos. 17-20) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following can be calculated based on Born-Haber cycle of formation of a lattice A+B- (s) from A(s) and 6 (g) ?
a)
Lattice energy of A+B- (s)
b)
Electron affinity of B (g)
c)
Hydration energy of A+(g)
d)
Ionisation energy of A (s).

|
Siddharth Chaudhary answered |
Born Haber cycle involves
Solubility of KCI is maximum in- a)CH3CH2OH
- b)CH3OCH3
- c)H2O
- d)CH3COOH
Correct answer is option 'C'. Can you explain this answer?
Solubility of KCI is maximum in
a)
CH3CH2OH
b)
CH3OCH3
c)
H2O
d)
CH3COOH

|
Parth Iyer answered |
KCI is an ionic compound. Force of attraction (F) between K+ 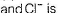
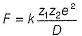

Greater the value of D, smaller the value of F, hence larger the solubility.
Note D for CH3OCH3 is least out of given solvents, thus solubility is minimum.
Greater the value of D, smaller the value of F, hence larger the solubility.
Note D for CH3OCH3 is least out of given solvents, thus solubility is minimum.
Chapter doubts & questions for Ionic Compounds - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Ionic Compounds - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup