All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Acid and Bases for EmSAT Achieve Exam
The acid present in curd is:a)Lactic acidb)Citric acidc)Oxalic acidd)Acetic acidCorrect answer is 'A'. Can you explain this answer?
|
|
Rahul Kapoor answered |
The acid present in curd is lactic acid.The increased acidity causes the milk proteins (casein) to tangle into solid masses, or curds. Milk that has been left to sour (raw milk alone or pasteurized milk with added lactic acid bacteria) will also naturally produce curds, and sour milk cheeses are produced this way.
Can you explain the answer of this question below:Select the substance which shows acidic behavior in its aqueous solution.- A:C6H12O6
- B:HCl
- C:C2H5OH
- D:Urea
The answer is b.
Select the substance which shows acidic behavior in its aqueous solution.
A:
C6H12O6
B:
HCl
C:
C2H5OH
D:
Urea
|
|
Alisha kapoor answered |
Acid is defined as the substance which dissociates and releases H+ ions in their aqueous states.
The equation for the dissociation of hydrochloric acid is given by:
HCl (aq.) gives H+ (aq.) + OH-(aq)
But molecules like alcohol and glucose and Urea do not produce H+ ions and thus are not considered as acids.
Name an acid which contains both oxygen and hydrogen?- a)Oxy acid
- b)Hydra acid
- c)Dilute acid
- d)Concentrated acid
Correct answer is option 'A'. Can you explain this answer?
Name an acid which contains both oxygen and hydrogen?
a)
Oxy acid
b)
Hydra acid
c)
Dilute acid
d)
Concentrated acid

|
Ameya Rane answered |
Oxy acid is an acid which contains both oxygen and hydrogen.
An oxyacid is an acid that contains an oxygen atom bonded to a hydrogen atom and at least one other element.
An oxyacid has the general structure X-O-H.
Which of the following does not conduct electricity?- a)Sodium hydroxide
- b)Rain water
- c)Hydrochloric acid
- d)Distilled water
Correct answer is option 'D'. Can you explain this answer?
Which of the following does not conduct electricity?
a)
Sodium hydroxide
b)
Rain water
c)
Hydrochloric acid
d)
Distilled water
|
|
Gaurav Kumar answered |
Distilled water do not conduct electricity. The reason is that a liquid conducts electricity is by the positively or negatively charged ions that are actually moving from one of the electrodes to the other, carrying charge (electricity) with them.
Lime water is- a)CaO
- b)Ca(OH)2
- c)CaCO3
- d)CaCI2
Correct answer is option 'B'. Can you explain this answer?
Lime water is
a)
CaO
b)
Ca(OH)2
c)
CaCO3
d)
CaCI2
|
|
Kiran Mehta answered |
Limewater is the common name for a dilute aqueous solution of calcium hydroxide.
Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). It is basic in nature with a pH of 12.4.
Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). It is basic in nature with a pH of 12.4.
Which of the property is not shown by bases?- a)Bases dissociate in water to give OH- ions
- b)Bases are soapy in touch
- c)Bases turn blue litmus red
- d)Bases are bitter in taste
Correct answer is option 'C'. Can you explain this answer?
Which of the property is not shown by bases?
a)
Bases dissociate in water to give OH- ions
b)
Bases are soapy in touch
c)
Bases turn blue litmus red
d)
Bases are bitter in taste
|
|
Arun Sharma answered |
Blue litmus paper is mainly used to test acids. It will turn red when it comes into contact with an acid. If it comes into contact with a substance that is basic or neutral, it will remain blue.
Acids when dissolved in water produce:
- a)Hydrogen and hydroxide ions
- b)Hydroxide ions
- c)Hydride ions
- d)Hydronium ions
Correct answer is option 'D'. Can you explain this answer?
Acids when dissolved in water produce:
a)
Hydrogen and hydroxide ions
b)
Hydroxide ions
c)
Hydride ions
d)
Hydronium ions

|
Snehal Sen answered |
Most acids release H+ ions in water, which combines with water molecule to produce hydronium (H3O+) ion.
Eg.
HCl(g)+H2O(l)→Cl−(aq)+H3O+(aq)
Can you explain the answer of this question below:Which of the following acid is a weak acid?- A:HCl
- B:H2SO4
- C:HNO3
- D:CH3COOH
The answer is d.
Which of the following acid is a weak acid?
A:
HCl
B:
H2SO4
C:
HNO3
D:
CH3COOH

|
Naina Chopra answered |
Acids like acetic acid ( CH3COOH ) dissociates partially or incompletely, releasing only some of its hydrogen atoms into the solution. Hence they are weak acid.
Which one will not change from red litmus to blue?a) NaClb) HClc) KOHd) LiOHCorrect answer is option 'B'. Can you explain this answer?
b) HCl
c) KOH
d) LiOH
Correct answer is option 'B'. Can you explain this answer?

|
Tarun Mehra answered |
Explanation: Since HCl is a base , so it turns red litmus to blue.
So option B is a correct answer.
The natural source of Acetic acid is:- a)Curd
- b)Oranges
- c)Tomatoes
- d)Vinegar
Correct answer is option 'D'. Can you explain this answer?
The natural source of Acetic acid is:
a)
Curd
b)
Oranges
c)
Tomatoes
d)
Vinegar
|
|
Krishna Iyer answered |
Option A: Lactic acid in curd
Option B: Citrus acid in Oranges
Option C: Oxalic acid is found in tomatoes
Option D: Acetic acid is found in vinegar
Option B: Citrus acid in Oranges
Option C: Oxalic acid is found in tomatoes
Option D: Acetic acid is found in vinegar
Thus, option D is correct.
Identify ‘X’ in the reaction: 2HCl + CuO → X + H2O- a)CuCl
- b)Cu(OH)2
- c)CuCl2
- d)HOCl
Correct answer is option 'C'. Can you explain this answer?
Identify ‘X’ in the reaction: 2HCl + CuO → X + H2O
a)
CuCl
b)
Cu(OH)2
c)
CuCl2
d)
HOCl
|
|
Vikram Kapoor answered |
When copper oxide and dilute hydrochloric acid are mixed the blue green solution is formed.
The reaction is :-
CuO + 2HCl → CuCl2 + H2O
The reaction is :-
CuO + 2HCl → CuCl2 + H2O
A fruit juice is tested for its pH value. What could be its possible pH if the colour is changed to yellow?- a)Between 6.5 and 7.5
- b)Less than 6.0
- c)7
- d)More than 7.5
Correct answer is option 'B'. Can you explain this answer?
A fruit juice is tested for its pH value. What could be its possible pH if the colour is changed to yellow?
a)
Between 6.5 and 7.5
b)
Less than 6.0
c)
7
d)
More than 7.5
|
|
Krishna Iyer answered |
Less than 6.0
pH range ( 5-6) shows up yellow colour on pH paper.
pH range ( 5-6) shows up yellow colour on pH paper.
The colour of phenolphthalein in acids is:- a)Colourless
- b)Red
- c)Pink
- d)Blue
Correct answer is option 'A'. Can you explain this answer?
The colour of phenolphthalein in acids is:
a)
Colourless
b)
Red
c)
Pink
d)
Blue
|
|
Krishna Iyer answered |
Phenolphthalein is often used as an indicator in acid–base titrations. For this application, it turns colourless in acidic solutions and magenta in basic solutions.
Tooth decay occurs when pH of mouth is below:- a)5.5
- b)7.5
- c)8.5
- d)10.5
Correct answer is option 'A'. Can you explain this answer?
Tooth decay occurs when pH of mouth is below:
a)
5.5
b)
7.5
c)
8.5
d)
10.5
|
|
Vikas Kumar answered |
When pH of mouth is lower than 5.5 tooth decay starts our tooth enamel is the hardest substance in our body but it is corroded when pH of mouth falls below 5.5 bacteria present in our mouth produce acid due to degradation of sugar and other food particles remainig in the mouth after eating.
Carbon dioxide is an example of:- a)Amphoteric oxide
- b)Acidic oxide
- c)Basic oxide
- d)Neutral oxide
Correct answer is option 'B'. Can you explain this answer?
Carbon dioxide is an example of:
a)
Amphoteric oxide
b)
Acidic oxide
c)
Basic oxide
d)
Neutral oxide
|
|
Gaurav Kumar answered |
Acid oxides is a complex chemical substance oxides, which form a salt with the chemical reactions with bases or basic oxides and do not react with acidic oxides.
Examples of acidic oxides can be:
CO2 (all known carbon dioxide), P2O5 - oxide of phosphorus (formed in air if burns white phosphorus), SO3 - oxide of sulfur (VI) is a substance used for sulfuric acid.
Examples of acidic oxides can be:
CO2 (all known carbon dioxide), P2O5 - oxide of phosphorus (formed in air if burns white phosphorus), SO3 - oxide of sulfur (VI) is a substance used for sulfuric acid.
Which of the following will be the strongest base?- a)pH = 11.5
- b)pH = 8.5
- c)pH = 13
- d)pH = 7.5
Correct answer is option 'C'. Can you explain this answer?
Which of the following will be the strongest base?
a)
pH = 11.5
b)
pH = 8.5
c)
pH = 13
d)
pH = 7.5
|
|
Vikram Kapoor answered |
Strong bases have very high pH values, usually in range 12 to 14.
The colour of anhydrous copper sulphate is:- a)White
- b)Blue
- c)Red
- d)Yellow
Correct answer is option 'A'. Can you explain this answer?
The colour of anhydrous copper sulphate is:
a)
White
b)
Blue
c)
Red
d)
Yellow
|
|
Rahul Kapoor answered |
The answer is b.
The crystals of hydrated copper sulphate salt are blue in colour. When heated, the salt loses its water of crystallization and turns white.
Which of the following is not an organic acid?- a)Acetic acid
- b)Sulphuric acid
- c)Tartaric acid
- d)Citric acid
Correct answer is option 'B'. Can you explain this answer?
Which of the following is not an organic acid?
a)
Acetic acid
b)
Sulphuric acid
c)
Tartaric acid
d)
Citric acid
|
|
Amit Kumar answered |
Sulfuric acid is inorganic because it doesn't contain carbon atoms bonded to oxygen, nitrogen and hydrogen atoms.
Name an element which is common to all acids?- a)Sulphur
- b)Chlorine
- c) Nitrogen
- d)Hydrogen
Correct answer is option 'D'. Can you explain this answer?
Name an element which is common to all acids?
a)
Sulphur
b)
Chlorine
c)
Nitrogen
d)
Hydrogen

|
Tarun Mehra answered |
An element which is common to all acids is Hydrogen .
So opttion D is correct option.
Find the odd one out:- a)Neutral salt : NaCl
- b)Acid salt : CuSO4.5H2O
- c) Basic salt: CuCO3.Cu(OH)2
- d)Nonhydrated salt: KNO3
Correct answer is option 'B'. Can you explain this answer?
Find the odd one out:
a)
Neutral salt : NaCl
b)
Acid salt : CuSO4.5H2O
c)
Basic salt: CuCO3.Cu(OH)2
d)
Nonhydrated salt: KNO3

|
Ashish Choudhary answered |
Explanation: CuSO4.5H20 is a hydrated salt. An example of acid salt is NaHCO3.
So option B is the correct answer.
Identify the type of reaction: HCl + NaOH → NaCl + H2O- a)Combination reaction
- b)Double decomposition reaction
- c)Decomposition reaction
- d)Neutralisation reaction
Correct answer is option 'D'. Can you explain this answer?
Identify the type of reaction: HCl + NaOH → NaCl + H2O
a)
Combination reaction
b)
Double decomposition reaction
c)
Decomposition reaction
d)
Neutralisation reaction
|
|
Krishna Iyer answered |
Reaction of a strong acid with strong base is called neutralization reaction which produces salt and water,
HCl + NaOH → NaCl + H2O
This equation is already balanced.
Which gas causes the bread or cake to rise making them soft and spongy?- a)CO2
- b)O2
- c)CO
- d)HCl
Correct answer is option 'A'. Can you explain this answer?
Which gas causes the bread or cake to rise making them soft and spongy?
a)
CO2
b)
O2
c)
CO
d)
HCl
|
|
Vikram Kapoor answered |
Carbon dioxide is released when baking soda is added to cake and this makes the cake to rise making them soft and spongy.
नम्नलिखित गद्यांशों को ध्यानपूर्वक पढ़कर नीचे दिए गए प्रश्नों के सही उत्तर विकल्पों में से चुनिए:रज्जो ने चार-पाँच आम अंजुली में लेकर मेरी ओर बढ़ा दिए। आम लेने के लिए मैंने हाथ बढ़ाया तो मेरी निगाह एक क्षण के लिए उसके हाथों पर ठिठक गई। गोरी-गोरी कलाइयों पर लाख की चूडिय़ाँ बहुत ही फब रही थीं।
बदलू ने मेरी दृष्टि देख ली और बोल पड़ा, यही आखिरी जोड़ा बनाया था ज्जमींदार साहब की बेटी के विवाह पर। दस आने पैसे मुझको दे रहे थे। मैंने जोड़ा नहीं दिया। कहा, शहर से ले आओ।प्रश्न - बदलू ने शमींदार को चूड़ियों का जोड़ा क्यों नहीं दिया? - a)सुंदर न होने के कारण
- b)अपनी बेटी को देने के कारण
- c)दाम अध्कि पाने के कारण
- d)कम दाम पाने के कारण
Correct answer is option 'D'. Can you explain this answer?
नम्नलिखित गद्यांशों को ध्यानपूर्वक पढ़कर नीचे दिए गए प्रश्नों के सही उत्तर विकल्पों में से चुनिए:
रज्जो ने चार-पाँच आम अंजुली में लेकर मेरी ओर बढ़ा दिए। आम लेने के लिए मैंने हाथ बढ़ाया तो मेरी निगाह एक क्षण के लिए उसके हाथों पर ठिठक गई। गोरी-गोरी कलाइयों पर लाख की चूडिय़ाँ बहुत ही फब रही थीं।
बदलू ने मेरी दृष्टि देख ली और बोल पड़ा, यही आखिरी जोड़ा बनाया था ज्जमींदार साहब की बेटी के विवाह पर। दस आने पैसे मुझको दे रहे थे। मैंने जोड़ा नहीं दिया। कहा, शहर से ले आओ।
बदलू ने मेरी दृष्टि देख ली और बोल पड़ा, यही आखिरी जोड़ा बनाया था ज्जमींदार साहब की बेटी के विवाह पर। दस आने पैसे मुझको दे रहे थे। मैंने जोड़ा नहीं दिया। कहा, शहर से ले आओ।
प्रश्न - बदलू ने शमींदार को चूड़ियों का जोड़ा क्यों नहीं दिया?
a)
सुंदर न होने के कारण
b)
अपनी बेटी को देने के कारण
c)
दाम अध्कि पाने के कारण
d)
कम दाम पाने के कारण
|
|
Geetika Shah answered |
D is the correct option.कम दाम पाने के कारण बदलू ने शमींदार को चूड़ियों का जोड़ा नहीं दिया.
Select the one which is wrongly mapped- a)Sodium carbonate – Washing soda
- b) Sodium chloride – common salt
- c)Calcium carbonate – slaked lime
- d) Sodium hydroxide – caustic soda
Correct answer is option 'C'. Can you explain this answer?
Select the one which is wrongly mapped
a)
Sodium carbonate – Washing soda
b)
Sodium chloride – common salt
c)
Calcium carbonate – slaked lime
d)
Sodium hydroxide – caustic soda

|
Subhankar Kapoor answered |
Calcium hydroxide is commonly referred as slaked lime.
The pH of a solution which is neither acidic nor basic is:- a)8
- b)5
- c)6
- d)7
Correct answer is 'D'. Can you explain this answer?
The pH of a solution which is neither acidic nor basic is:
a)
8
b)
5
c)
6
d)
7
|
|
Pooja Shah answered |
A solution that is neither acidic nor basic is neutral and has a pH of 7.
Aqueous solution of sodium hydroxide turns blue litmus:- a)Red
- b)No change
- c)Colourless
- d)Pink
Correct answer is option 'B'. Can you explain this answer?
Aqueous solution of sodium hydroxide turns blue litmus:
a)
Red
b)
No change
c)
Colourless
d)
Pink

|
Sagar Rane answered |
Since Sodium hydroxide is a base and thus it has no effect on a blue litmus paper but it changes red litmus to blue.
Which of the following property is not shown by acids ?- a)Acids dissociate in water to give OH- ions.
- b)Acids are corrosive.
- c)Acids dissociate in water to give H+ ions.
- d)Acids are sour in taste.
Correct answer is option 'A'. Can you explain this answer?
Which of the following property is not shown by acids ?
a)
Acids dissociate in water to give OH- ions.
b)
Acids are corrosive.
c)
Acids dissociate in water to give H+ ions.
d)
Acids are sour in taste.
|
|
Arun Sharma answered |
Acids dissociate in water to give H+ ions and not OH-.
Which of the following salt is basic in nature?- a)Aluminium chloride
- b)Sodium acetate
- c)Sodium chloride
- d)Potassium nitrate
Correct answer is option 'B'. Can you explain this answer?
Which of the following salt is basic in nature?
a)
Aluminium chloride
b)
Sodium acetate
c)
Sodium chloride
d)
Potassium nitrate
|
|
Krishna Iyer answered |
Sodium acetate (CH3COONa) is a salt in solid state and can't be regarded as an acid or base in anhydrous or molten form.
Although, being an ionic compound, sodium acetate dissociates in water to produce sodium ion Na+ and acetate ion CH3COO-. These ions react with water producing NaOH and CH3COOH.
Now, as NaOH is a strong base and CH3COOH is a weak acid, the resultant solution is basic in nature.
Hence, sodium acetate is basic in aqueous medium.
Although, being an ionic compound, sodium acetate dissociates in water to produce sodium ion Na+ and acetate ion CH3COO-. These ions react with water producing NaOH and CH3COOH.
Now, as NaOH is a strong base and CH3COOH is a weak acid, the resultant solution is basic in nature.
Hence, sodium acetate is basic in aqueous medium.
Which of following acid is present in tamarind?- a)Ethanoic acid
- b)Sulphuric acid
- c)Nitric acid
- d)Tartaric acid
Correct answer is option 'D'. Can you explain this answer?
Which of following acid is present in tamarind?
a)
Ethanoic acid
b)
Sulphuric acid
c)
Nitric acid
d)
Tartaric acid
|
|
Arun Sharma answered |
The acid that is present in tamarind is tartaric acid, which is a white crystalline organic acid.
Calcium sulphate hemihydrate is known as:- a)Plaster of Paris
- b)Soda ash
- c)Washing soda
- d)Gypsum
Correct answer is option 'A'. Can you explain this answer?
Calcium sulphate hemihydrate is known as:
a)
Plaster of Paris
b)
Soda ash
c)
Washing soda
d)
Gypsum
|
|
Rajiv Gupta answered |
The name plaster of paris (POP) is derived as the calcium sulphate hemi hydrates are found in large amount deposited in the Montmartre hill in Paris
Plaster of Paris is obtained by heating gypsum or calcium sulphate dihydrate to about 140-180 degree Celsius.
When heated to such a temperature, gypsum forms Plaster of Paris.
The name is derived from the large deposits of gypsum in the Montmartre hill in Paris.
Which of the following is an olfactory indicator?- a)Litmus
- b)Phenolphthalein
- c)Onion
- d)Methyl orange
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an olfactory indicator?
a)
Litmus
b)
Phenolphthalein
c)
Onion
d)
Methyl orange
|
|
Amit Sharma answered |
An olfactory indicator is a material whose smell varies reliant on whether it is mixed with an acidic or basic solution. Olfactory indicators mainly used in laboratory to test whether a solution is a base or an acid. Onion is an example of olfactory indicators.
The acid present in curd is:- a)Lactic acid
- b)Citric acid
- c)Oxalic acid
- d)Acetic acid
Correct answer is 'A'. Can you explain this answer?
The acid present in curd is:
a)
Lactic acid
b)
Citric acid
c)
Oxalic acid
d)
Acetic acid

|
Baishali Kulkarni answered |
The acid present in curd is lactic acid
Bee-sting contains:- a)Methanoic acid
- b)Tartaric acid
- c)Acetic acid
- d)Ethanoic acid
Correct answer is option 'A'. Can you explain this answer?
Bee-sting contains:
a)
Methanoic acid
b)
Tartaric acid
c)
Acetic acid
d)
Ethanoic acid
|
|
Rohan Kapoor answered |
Bee sting venom is acidic and so its effects can be neutralised with bicarbonate of soda or alkali and this reaction reduces the pain. The facts are that: Bee venom contains formic acid (also known as methanoic acid) but this is not the single active ingredient that causes the pain from a bee sting.
Red cabbage indicator turns _______ in basic solutions.- a)Pink
- b)Green
- c)Blue
- d)Red
Correct answer is 'B'. Can you explain this answer?
Red cabbage indicator turns _______ in basic solutions.
a)
Pink
b)
Green
c)
Blue
d)
Red

|
Ruba answered |
This is a natural indicator which are found in the nature of the plants
Which of the following substances is used for supporting fractured bones?- a)Na2SO4
- b)CaSO4
- c)CaSO4.2H2O
- d)CaSO4.1/2 H2O
Correct answer is option 'D'. Can you explain this answer?
Which of the following substances is used for supporting fractured bones?
a)
Na2SO4
b)
CaSO4
c)
CaSO4.2H2O
d)
CaSO4.1/2 H2O
|
|
Vikas Kumar answered |
Chemical name of the powder: Calcium Sulphate Hemihydrate {also known as Plaster of Paris}
Its formula: 2CaSO4.1/2 H2O
when this white powder is mixed with water CaSO4.1/2 H2O + 1 1/2 H2O ----> CaSO4.2H2O
Marble chips reacts with a solution to produce a gas which turns lime water milky. So the solution contains:
- a)Na2SO4
- b)H2SO4
- c)K2SO4
- d)none of these
Correct answer is option 'B'. Can you explain this answer?
Marble chips reacts with a solution to produce a gas which turns lime water milky. So the solution contains:
a)
Na2SO4
b)
H2SO4
c)
K2SO4
d)
none of these
|
|
Rajiv Gupta answered |
Marble chips are the substances that have the formula CaCO3
Calcium carbonate reacts with sulphuric acid to form calcium sulphate and carbon dioxide which turns lime water milky.
Calcium carbonate reacts with sulphuric acid to form calcium sulphate and carbon dioxide which turns lime water milky.
CaCO3 + H2SO4 → CaSO4 + CO2 + H2O
Acids turn blue litmus :- a)Blue
- b)Red
- c)colourless
- d)Pink
Correct answer is 'B'. Can you explain this answer?
Acids turn blue litmus :
a)
Blue
b)
Red
c)
colourless
d)
Pink

|
Ameya Rane answered |
Blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic or alkaline conditions
A student dipped a pH paper in an unknown liquid. Orange colour was obtained. The unknown solution can be:- a)Acetic acid
- b)Ethanoic acid
- c)Sodium carbonate
- d)Both acetic acid and ethanoic acid
Correct answer is option 'D'. Can you explain this answer?
A student dipped a pH paper in an unknown liquid. Orange colour was obtained. The unknown solution can be:
a)
Acetic acid
b)
Ethanoic acid
c)
Sodium carbonate
d)
Both acetic acid and ethanoic acid
|
|
Kiran Mehta answered |
Both acetic acid and ethanoic acid can turn the pH paper orange. Sodium carbonate is basic in nature.
Assertion : Weak acids have low electrical conductivity.Reason : Strong acids and weak acids have equal concentration of hydrogen ions in their solutions.- a)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Assertion (A) is false but reason (R) is true.
- e)Both Assertion and Reason are false.
Correct answer is option 'C'. Can you explain this answer?
Assertion : Weak acids have low electrical conductivity.
Reason : Strong acids and weak acids have equal concentration of hydrogen ions in their solutions.
a)
Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
b)
Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
c)
Assertion (A) is true but reason (R) is false.
d)
Assertion (A) is false but reason (R) is true.
e)
Both Assertion and Reason are false.
|
|
Radha Iyer answered |
Assertion (A) is true but reason (R) is false.
Bases generate hydroxide__________ ions in water.
- a)H-
- b)H+
- c)OH-
- d)OH+
Correct answer is option 'C'. Can you explain this answer?
Bases generate hydroxide__________ ions in water.
a)
H-
b)
H+
c)
OH-
d)
OH+
|
|
Krishna Iyer answered |
Bases generate hydroxide ions or OH- ions in water. Thus option C is correct
Which one of the following is acidic/slightly acidic?- a)Lemon juice
- b)Tomatoes
- c)Milk
- d)All
Correct answer is option 'D'. Can you explain this answer?
Which one of the following is acidic/slightly acidic?
a)
Lemon juice
b)
Tomatoes
c)
Milk
d)
All
|
|
Rohit Jain answered |
Option A: Lemon juice in its natural state is acidic with a pH of about 2, but once metabolized it actually becomes alkaline with a pH well above 7. So, outside the body, anyone can see that lemon juice is very acidic.
Option B: Tomatoes are on the low end of acidic foods. An alkaline diet is not that you can not have any foods that are acidic but a balance of both alkaline and acidic.
Option C: Milk is actually pretty close to neutral with a pH of 6.8 (slightly acidic). It can become acidic if bacteria break down the milk sugar and produce lactic acid. This is what causes milk to sour.
Thus, option D is correct.
We should never add water to acid because:- a)The reaction is endothermic.
- b)The reaction is slow.
- c)The reaction is exothermic.
- d)The reaction doesn’t take place.
Correct answer is 'C'. Can you explain this answer?
We should never add water to acid because:
a)
The reaction is endothermic.
b)
The reaction is slow.
c)
The reaction is exothermic.
d)
The reaction doesn’t take place.
|
|
Amit Sharma answered |
If we add water to acid, the reaction would become highly exothermic and lots of heat would be generated which would splash on your face and cause severe burns.
Plaster of Paris is prepared by heating which of the following compounds?- a)Limestone
- b)Soda ash
- c)Lime
- d)Gypsum
Correct answer is option 'D'. Can you explain this answer?
Plaster of Paris is prepared by heating which of the following compounds?
a)
Limestone
b)
Soda ash
c)
Lime
d)
Gypsum
|
|
Krishna Iyer answered |
Gypsum is calcium sulphate dihydrate. The chemical formula of gypsum is CaSO4·2H2O
Plaster of paris is prepared by heating gypsum to a temperature of 373K.When gypsum is heated to a temperature of 373k ,It loses three-Fourths of its water of crystallisation and forms plaster of paris.
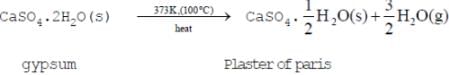
Plaster of paris is prepared by heating gypsum to a temperature of 373K.When gypsum is heated to a temperature of 373k ,It loses three-Fourths of its water of crystallisation and forms plaster of paris.

Four student (a), (b), (c) and (d) measured the pH values of water, lemon juice and sodium bicarbonate solution. What is the correct decreasing order of pH values?- a)Water > lemon juice > sodium bicarbonate
- b)Water > sodium bicarbonate > lemon juice
- c)Lemon juice > water > sodium bicarbonate
- d)Sodium bicarbonate > water > lemon juice
Correct answer is option 'D'. Can you explain this answer?
Four student (a), (b), (c) and (d) measured the pH values of water, lemon juice and sodium bicarbonate solution. What is the correct decreasing order of pH values?
a)
Water > lemon juice > sodium bicarbonate
b)
Water > sodium bicarbonate > lemon juice
c)
Lemon juice > water > sodium bicarbonate
d)
Sodium bicarbonate > water > lemon juice

|
Ashwani Mishra answered |
1.Sodium bicarbonate (as it is a base and has pH value more than 7) .
2. Water (as it is neutral and has pH value as 7) .
3. Lemon juice (it is acidic and the pH value is less than 7).
2. Water (as it is neutral and has pH value as 7) .
3. Lemon juice (it is acidic and the pH value is less than 7).
Acids change the colour of methyl orange to:- a)Colourless
- b)Pink/Red
- c)Blue
- d)Purple
Correct answer is option 'B'. Can you explain this answer?
Acids change the colour of methyl orange to:
a)
Colourless
b)
Pink/Red
c)
Blue
d)
Purple
|
|
Vikas Kumar answered |
Methyl orange is a pH indicator frequently used in titration because of its clear and distinct colour variance at different pH values. Methyl orange shows pink colour in acidic medium and yellow colour in basic medium. Because it changes colour at the pH of a mid strength acid, it is usually used in titration for acids. Unlike a universal indicator, methyl orange does not have a full spectrum of colour change, but it has a sharp end point.
Which of the following compound can turn blue litmus solution red?- a)CH3CHO
- b)NaOH
- c)CH3OCH3
- d)CH3COOH
Correct answer is 'D'. Can you explain this answer?
Which of the following compound can turn blue litmus solution red?
a)
CH3CHO
b)
NaOH
c)
CH3OCH3
d)
CH3COOH
|
|
Krishna Iyer answered |
Acid convert blue litmus solution to Red. HCHO, CH3CHO are aldehydes. HCOOH, CH3COOH are carboxylic acids. CH3OH and C2H5OH are alcohols. Out of these only carboxyhc acids would turn blue litmus solution red. So HCOOH and CH3COOH would turn blue litmus solution red.
'Litmus', a natural dye, is an extract of which of the following?- a)China rose(Gudhal)
- b)Beetroot
- c)Lichen
- d)Blueberries(Jamun)
Correct answer is option 'C'. Can you explain this answer?
'Litmus', a natural dye, is an extract of which of the following?
a)
China rose(Gudhal)
b)
Beetroot
c)
Lichen
d)
Blueberries(Jamun)
|
|
Krishna Iyer answered |
Litmus', a natural dye extracted from Lichen which is a composite organism emerges from algae.
A colourless and odourless gas is liberated when hydrochloric acid is added to a solution of washing soda. The name of the gas is:- a)Nitrogen dioxide
- b)Carbon dioxide
- c)Sulphur trioxide
- d)Sulphur dioxide
Correct answer is option 'B'. Can you explain this answer?
A colourless and odourless gas is liberated when hydrochloric acid is added to a solution of washing soda. The name of the gas is:
a)
Nitrogen dioxide
b)
Carbon dioxide
c)
Sulphur trioxide
d)
Sulphur dioxide
|
|
Kshitij naidu answered |
Washing soda is chemically sodium carbonate, which is a basic salt. When dilute HCl is added to washing soda, acid base neutralisation reaction takes place and sodium chloride is formed along with water and carbon dioxide. Following is the chemical equation for the reaction
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
Hence, the correct answer is (b) carbon dioxide.
pH of distilled water is:- a)7
- b)6
- c)5
- d)8
Correct answer is option 'A'. Can you explain this answer?
pH of distilled water is:
a)
7
b)
6
c)
5
d)
8

|
Rachit Agrah.ari answered |
PH of distilled water is 7...
The compounds obtained on heating baking soda are:- a)Hydrochloric acid and water
- b)Sodium carbonate, water and carbon dioxide
- c)Sodium bicarbonate, water and carbon dioxide
- d)Sulphur dioxide and sodium carbonate
Correct answer is option 'B'. Can you explain this answer?
The compounds obtained on heating baking soda are:
a)
Hydrochloric acid and water
b)
Sodium carbonate, water and carbon dioxide
c)
Sodium bicarbonate, water and carbon dioxide
d)
Sulphur dioxide and sodium carbonate
|
|
Amit Kumar answered |
Sodium hydrogen carbonate (also known as sodium bicarbonate or bicarbonate of soda) has the chemical formula NaHCO3 . When it is heated above about 80°C it begins to break down, forming sodium carbonate, water and carbon dioxide.
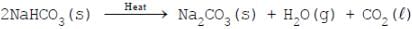
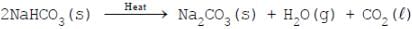
Chapter doubts & questions for Acid and Bases - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Acid and Bases - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









