All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Enthalpy for EmSAT Achieve Exam
Specific heat of water is 4.184 JK-1g-1, Rise in temperature when 1 kJ of heat is absorbed by 2 moles of H2O is- a)119.5o
- b)0.37o
- c)0.12o
- d)6.64o
Correct answer is option 'D'. Can you explain this answer?
Specific heat of water is 4.184 JK-1g-1, Rise in temperature when 1 kJ of heat is absorbed by 2 moles of H2O is
a)
119.5o
b)
0.37o
c)
0.12o
d)
6.64o
|
|
Rajesh Gupta answered |
We have,
Q = ms∆T
1000 = 2*18*4.184*∆T
∆T = 6.63 K
Q = ms∆T
1000 = 2*18*4.184*∆T
∆T = 6.63 K
Heat of hydrogenation of ethene is x1 and that of benzene is x2. The resonance energy of benzene is- a)x1 — x2
- b)x1 + x2
- c)3x1 — x2
- d)x1 - 3x2
Correct answer is option 'C'. Can you explain this answer?
Heat of hydrogenation of ethene is x1 and that of benzene is x2. The resonance energy of benzene is
a)
x1 — x2
b)
x1 + x2
c)
3x1 — x2
d)
x1 - 3x2
|
|
Anaya Patel answered |
Energy of ethene =x1
Number of ethene in benzene=3.
Therefore, Observed energy of hydrogenation of benzene =3x1
Calculated energy of hydrogenation of benzene =x2 (Given)
Therefore,
Resonance energy = observed - calculated =3x1−x2
Number of ethene in benzene=3.
Therefore, Observed energy of hydrogenation of benzene =3x1
Calculated energy of hydrogenation of benzene =x2 (Given)
Therefore,
Resonance energy = observed - calculated =3x1−x2
What happens to the ions, when an ionic compound dissolves in a solvent?- a)They leave their ordered position in the crystal lattice and gets hydrated.
- b)They leave their ordered position in the crystal lattice and doesn’t hydrate.
- c)They doesn’t leave their ordered position in the crystal lattice and gets hydrated.
- d)None of the above.
Correct answer is option 'A'. Can you explain this answer?
What happens to the ions, when an ionic compound dissolves in a solvent?
a)
They leave their ordered position in the crystal lattice and gets hydrated.
b)
They leave their ordered position in the crystal lattice and doesn’t hydrate.
c)
They doesn’t leave their ordered position in the crystal lattice and gets hydrated.
d)
None of the above.
|
|
Pooja Shah answered |
When an ionic compound is dissolved in the solvent, the compound dissociate into ions and due to external medium i. e. solvent it leave its original position in crystal lattice n gets hydrated..
Eg. NaCl(aq) → Na+(aq) + Cl-(aq)
Eg. NaCl(aq) → Na+(aq) + Cl-(aq)
When 4.0 mL of 2.0 N solution of weak acid is neutralised by a dilute aqueous solution of sodium hydroxide, 64 cal of heat is liberated. What is the heat of neutralisation in cal/milliequivalent?
Correct answer is '8'. Can you explain this answer?
When 4.0 mL of 2.0 N solution of weak acid is neutralised by a dilute aqueous solution of sodium hydroxide, 64 cal of heat is liberated. What is the heat of neutralisation in cal/milliequivalent?
|
|
Riya Banerjee answered |
Milliequivalents of the acid taken = 4×2 =8.
neutralization of 8 meq liberated 64 cal heat.
So the heat of neutralization is 64/8 = 8cal/meq.
neutralization of 8 meq liberated 64 cal heat.
So the heat of neutralization is 64/8 = 8cal/meq.
Direction (Q. Nos. 12-15) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d).Passage IOctane (C8H18), a primary constituent of gasoline, burns in air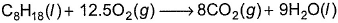 A 1.00 g sample of octane is burned in a constant volume calorimeter containing 1.20 kg of water. The temperature of water and the bomb rises by 8.2 K. The heat capacity of the bomb is 837.0 JK-1. Molar heat capacity of water is 4.184 J g-1 K-1. Initial temperature is 298 K. Q. ΔCU° (per mol of C8H18) is
A 1.00 g sample of octane is burned in a constant volume calorimeter containing 1.20 kg of water. The temperature of water and the bomb rises by 8.2 K. The heat capacity of the bomb is 837.0 JK-1. Molar heat capacity of water is 4.184 J g-1 K-1. Initial temperature is 298 K. Q. ΔCU° (per mol of C8H18) is- a)-48.03 kJ
- b)+48.03 kJ
- c)-5475.8 kJ
- d)+5475.8 kJ
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 12-15) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d).
Passage I
Octane (C8H18), a primary constituent of gasoline, burns in air
A 1.00 g sample of octane is burned in a constant volume calorimeter containing 1.20 kg of water. The temperature of water and the bomb rises by 8.2 K. The heat capacity of the bomb is 837.0 JK-1. Molar heat capacity of water is 4.184 J g-1 K-1. Initial temperature is 298 K.
Q. ΔCU° (per mol of C8H18) is
a)
-48.03 kJ
b)
+48.03 kJ
c)
-5475.8 kJ
d)
+5475.8 kJ
|
|
Raghav Bansal answered |

The standard enthalpies of formation of CO2(g), H2O (l) and glucose (s) at 25°C are - 400 kJ mol-1, - 300 kJ mol-1 and - 1300 kJ mol-1 respectively. The standard enthalpy of combustion per gram of glucose at 25°C is[JEE Advanced 2013]- a)2900 kJ
- b)-2900 kJ
- c)-16.11 kJ
- d)+16.11 kJ
Correct answer is option 'C'. Can you explain this answer?
The standard enthalpies of formation of CO2(g), H2O (l) and glucose (s) at 25°C are - 400 kJ mol-1, - 300 kJ mol-1 and - 1300 kJ mol-1 respectively. The standard enthalpy of combustion per gram of glucose at 25°C is
[JEE Advanced 2013]
a)
2900 kJ
b)
-2900 kJ
c)
-16.11 kJ
d)
+16.11 kJ
|
|
Anjana Sharma answered |

Direction (Q. No. 10) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.Q.Statement I : Based on the following thermodynamic data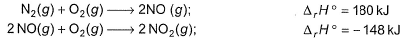 NO2 is more stable than NO.Statement II : NO (g) is an endothermic compound while, NO2(g) is an exothermic compound.
NO2 is more stable than NO.Statement II : NO (g) is an endothermic compound while, NO2(g) is an exothermic compound.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. No. 10) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q.
Statement I : Based on the following thermodynamic data
NO2 is more stable than NO.
Statement II : NO (g) is an endothermic compound while, NO2(g) is an exothermic compound.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Knowledge Hub answered |
Reaction number 1 is less stable because heat is not released thus the product side of reaction gains energy which get stored in the product and decreases its stability
While in reaction number 2, the reaction formed is more stable because energy is released and so the product is in a more stable state.
Now there is no such endothermic or exothermic compound , the reaction is exothermic or endothermic and not the product.
While in reaction number 2, the reaction formed is more stable because energy is released and so the product is in a more stable state.
Now there is no such endothermic or exothermic compound , the reaction is exothermic or endothermic and not the product.
The heat capacity ratio, r was determined for cyanogen as 1.177. Thus, Cp for this gas is- a)55.3 JK-1 mol-1
- b)33.26 JK-1 mol-1
- c)8.314 JK-1 mol-1
- d)24.94 JK-1 mol-1
Correct answer is option 'A'. Can you explain this answer?
The heat capacity ratio, r was determined for cyanogen as 1.177. Thus, Cp for this gas is
a)
55.3 JK-1 mol-1
b)
33.26 JK-1 mol-1
c)
8.314 JK-1 mol-1
d)
24.94 JK-1 mol-1

|
Infinity Academy answered |
γ = 1.177
CV = R/γ-1
= 8.314/0.177 = 46.97
Also γ = CP/CV
1.177 = CP/46.97
Or CP = 55.28
CV = R/γ-1
= 8.314/0.177 = 46.97
Also γ = CP/CV
1.177 = CP/46.97
Or CP = 55.28
Among the following enthalpies, which is always less than zero?- a)ΔcH°
- b)ΔsubH°
- c)ΔmixH°
- d)ΔfH°
Correct answer is option 'A'. Can you explain this answer?
Among the following enthalpies, which is always less than zero?
a)
ΔcH°
b)
ΔsubH°
c)
ΔmixH°
d)
ΔfH°
|
|
Neha Joshi answered |
The symbol represents enthalpy of combustion which is always exothermic. For exothermic rxn delta H is always -ve.
The ΔrH° for CO2(g), CO(g) , and H2O(g) are - 393.5°, - 110.5 and - 241.8 kJ mol-1, respectively. Thus, ΔrH° for the reaction (in kJ)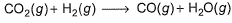 [IITJEE 2000]
[IITJEE 2000]- a)+524.1
- b)+41.2
- c)-262.5
- d)-41.2
Correct answer is option 'B'. Can you explain this answer?
The ΔrH° for CO2(g), CO(g) , and H2O(g) are - 393.5°, - 110.5 and - 241.8 kJ mol-1, respectively. Thus, ΔrH° for the reaction (in kJ)
[IITJEE 2000]
a)
+524.1
b)
+41.2
c)
-262.5
d)
-41.2
|
|
Krishna Iyer answered |
∆H= ∆HCO+∆HH2O-∆HCO2 (as ΔH for H2 is 0)
= -110.5-241.8+393.5 = +41.2 kJ mol-1
= -110.5-241.8+393.5 = +41.2 kJ mol-1
The dissociation energy of CH4 and C2H6 to convert them into gaseous atoms are 360 and 620 kcal mol-1 respectively. Thus, bond energy of (C—C) bond is- a)260 kcal mol-1
- b)180 kcal mol-1
- c)130 kcal mol-1
- d)80 kcal mol-1
Correct answer is option 'D'. Can you explain this answer?
The dissociation energy of CH4 and C2H6 to convert them into gaseous atoms are 360 and 620 kcal mol-1 respectively. Thus, bond energy of (C—C) bond is
a)
260 kcal mol-1
b)
180 kcal mol-1
c)
130 kcal mol-1
d)
80 kcal mol-1
|
|
Krishna Iyer answered |
In CH4 there are 4-C-H bonds.
Let C-H bond energy be X. So 4X = 360. or X = 90.
Now, in C2H6 there are 6 C-H bonds and 1C-C bond.
Let C-C B. E be y.
So 6X + Y = 620. or Y = 620-6×90 = 80.
Let C-H bond energy be X. So 4X = 360. or X = 90.
Now, in C2H6 there are 6 C-H bonds and 1C-C bond.
Let C-C B. E be y.
So 6X + Y = 620. or Y = 620-6×90 = 80.
The standard enthalpies of formation of SF6 (g), S (g)and F (g) are -1100, + 275 and + 80 kJ mol-1. Thus, average bond energy of (S—F) in SF6 is- a)309.16 kJ mol-1
- b)1855 kJ mol-1
- c)11.130 x 103 kJ mol-1
- d)-309.16 kJ mol-1
Correct answer is option 'A'. Can you explain this answer?
The standard enthalpies of formation of SF6 (g), S (g)and F (g) are -1100, + 275 and + 80 kJ mol-1. Thus, average bond energy of (S—F) in SF6 is
a)
309.16 kJ mol-1
b)
1855 kJ mol-1
c)
11.130 x 103 kJ mol-1
d)
-309.16 kJ mol-1
|
|
Suresh Reddy answered |
S(g) + 6F(g) → SF6(g)
∆rH = ∑∆Hreactant - ∑∆Hproduct
= (275+6 80) - (-1100)
= 1855
So, bond energy of (S—F) = 1855/6 = 309.16 kJ mol-1
∆rH = ∑∆Hreactant - ∑∆Hproduct
= (275+6 80) - (-1100)
= 1855
So, bond energy of (S—F) = 1855/6 = 309.16 kJ mol-1
Which of the following reactions defines ΔfH° ?- a)
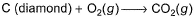
- b)
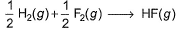
- c)
- d)
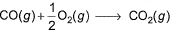
Correct answer is option 'B'. Can you explain this answer?
Which of the following reactions defines ΔfH° ?
a)
b)
c)
d)
|
|
Raghav Bansal answered |
For ∆H formation we have to look for following:
1. formation of "1 mole" of substance.
2. from "stable" constituting elements.
1. formation of "1 mole" of substance.
2. from "stable" constituting elements.
In first option 1 mole of Co2 should be formed from C(graphite) for enthalpy of formation to be defined.
In third option 2 moles of NH3 is formed instead of 1 mole. So incorrect.
In fourth option Co2 is not formed from constituent elements I.e C(gr) and O2(g) so it is not enthalpy of formation.
In third option 2 moles of NH3 is formed instead of 1 mole. So incorrect.
In fourth option Co2 is not formed from constituent elements I.e C(gr) and O2(g) so it is not enthalpy of formation.
ΔfU° of formation of CH4(g) at a certain temperature is - 393 kJ mol-1. The value of ΔfH° is- a)zero
- b)
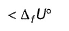
- c)
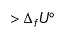
- d)
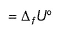
Correct answer is option 'C'. Can you explain this answer?
ΔfU° of formation of CH4(g) at a certain temperature is - 393 kJ mol-1. The value of ΔfH° is
a)
zero
b)
c)
d)
|
|
Pooja Shah answered |
The balanced equation for combustion of methane is:
CH4(g)+2O2(g)→CO2(g)+2H2O(l)Here, Δng=1−3=−2
ΔH0=ΔU0+ΔngRT
ΔH0 =−393−2RT
∴ΔH0<ΔU0
CH4(g)+2O2(g)→CO2(g)+2H2O(l)Here, Δng=1−3=−2
ΔH0=ΔU0+ΔngRT
ΔH0 =−393−2RT
∴ΔH0<ΔU0
Enthalpy change on mixing 100 mL of 1 M Ca(OH)2 with 100 mL of 1 M H2SO4 is- a)-13.7 kcal
- b)-27.4 kcal
- c)-1.37 kcal
- d)-2.74 kcal
Correct answer is option 'D'. Can you explain this answer?
Enthalpy change on mixing 100 mL of 1 M Ca(OH)2 with 100 mL of 1 M H2SO4 is
a)
-13.7 kcal
b)
-27.4 kcal
c)
-1.37 kcal
d)
-2.74 kcal
|
|
Pooja Shah answered |
For reaction of 1 mole of H+ and 1 mole of OH-, -13.6 kcal energy is released.
100 mL of 1 M Ca(OH)2 = moles = molarity volume = 0.1× 1 = 0.1
Moles of OH- = 0.2
100 mL of 1 M H2SO4= moles = molarity volume = 0.1× 1 = 0.1
Moles of H+ = 0.2
SO 0.2 moles of H+ and OH- reacts.
Energy released, 0.2 ×13.6 =2.74 kcal.
100 mL of 1 M Ca(OH)2 = moles = molarity volume = 0.1× 1 = 0.1
Moles of OH- = 0.2
100 mL of 1 M H2SO4= moles = molarity volume = 0.1× 1 = 0.1
Moles of H+ = 0.2
SO 0.2 moles of H+ and OH- reacts.
Energy released, 0.2 ×13.6 =2.74 kcal.
Sulphur (2.56 g) is burned in a constant volume calorimeter with excess O2(g). The temperature increases from 21.25°C to 26.72°C . The bomb has a heat capacity of 923 JK-1. Calorimeter contains 815 g of water. Thus, change in internal energy per mole of SO2 formed for the reaction is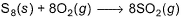 (specific heat of water is 4.184 JK-1g-1.)
(specific heat of water is 4.184 JK-1g-1.)- a)- 296.27 kJ
- b)+ 296.27 kJ
- c)- 2370.13 kJ
- d)+ 2370.13 kJ
Correct answer is option 'A'. Can you explain this answer?
Sulphur (2.56 g) is burned in a constant volume calorimeter with excess O2(g). The temperature increases from 21.25°C to 26.72°C . The bomb has a heat capacity of 923 JK-1. Calorimeter contains 815 g of water. Thus, change in internal energy per mole of SO2 formed for the reaction is
(specific heat of water is 4.184 JK-1g-1.)
a)
- 296.27 kJ
b)
+ 296.27 kJ
c)
- 2370.13 kJ
d)
+ 2370.13 kJ

|
Dhanush Kumar answered |
Therila
Assuming the composition of air to be X (N2) = 0.80, X (O2) = 0.18 and X (CO2) = 0.02, molar heat capacity of air at constant pressure is- a)3.50R
- b)4.00R
- c)2.50R
- d)3.51R
Correct answer is option 'D'. Can you explain this answer?
Assuming the composition of air to be X (N2) = 0.80, X (O2) = 0.18 and X (CO2) = 0.02, molar heat capacity of air at constant pressure is
a)
3.50R
b)
4.00R
c)
2.50R
d)
3.51R
|
|
Neha Joshi answered |
(Cp)mixture = (µ1Cp1+µ2Cp2+µ3Cp3+.........)/µ1+µ2+µ3+......... where µ is the no of moles.
Or (Cp)mixture = ƞ1Cp1+ƞ2Cp2+ƞ3Cp3+............. Where ƞ is the corr. mole fraction.
On putting the values, we have
Cpmixture= ( 7/2*0.8 +7/2*0.18 +4*0.02)R
=3.15R
Or (Cp)mixture = ƞ1Cp1+ƞ2Cp2+ƞ3Cp3+............. Where ƞ is the corr. mole fraction.
On putting the values, we have
Cpmixture= ( 7/2*0.8 +7/2*0.18 +4*0.02)R
=3.15R
Based on the following reactions,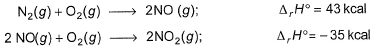 Q. Heat of formation of NO2 (in kcal) is ........
Q. Heat of formation of NO2 (in kcal) is ........
Correct answer is '4'. Can you explain this answer?
Based on the following reactions,
Q. Heat of formation of NO2 (in kcal) is ........

|
Lohit Matani answered |
N2(g) + O2(g) → 2NO ----- (i) ∆rH° = 43 kcal
2NO(g) + O2(g) → 2NO2(g) -----(ii) ∆rH° = -35 kcal
Enthalpy of formation of NO2 means we need to have 1 mole of NO2 from its constituting elements.
We will have (i)/2 + (ii)/2
½ N2(g) + O2(g) → NO2(g) ∆fH° = 4 kcal
2NO(g) + O2(g) → 2NO2(g) -----(ii) ∆rH° = -35 kcal
Enthalpy of formation of NO2 means we need to have 1 mole of NO2 from its constituting elements.
We will have (i)/2 + (ii)/2
½ N2(g) + O2(g) → NO2(g) ∆fH° = 4 kcal
Ethanol can undergo decomposition to form two sets of products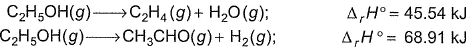 Q. If the molar ratio of C2H4 to CH3CHO is 8 : 1 in a set of product gases, then, energy involved in the decomposition process is
Q. If the molar ratio of C2H4 to CH3CHO is 8 : 1 in a set of product gases, then, energy involved in the decomposition process is- a)14.31 KJ
- b)57.23 KJ
- c)48.14 KJ
- d)66.31 KJ
Correct answer is option 'C'. Can you explain this answer?
Ethanol can undergo decomposition to form two sets of products
Q. If the molar ratio of C2H4 to CH3CHO is 8 : 1 in a set of product gases, then, energy involved in the decomposition process is
a)
14.31 KJ
b)
57.23 KJ
c)
48.14 KJ
d)
66.31 KJ
|
|
Lavanya Menon answered |
A/Q molar ratio of C2H4 and CH3CHO is 8:1
So from eqn 1
∆H = 8 45.54
= 364.32 kJ
Similarly from eqn
∆H = 68.91 1
= 68.91 kJ
Total ∆H = 364.32+68.91
Therefore, enthalpy change per mole of C2H5OH used 364.32+68.91/9
= 48.14 kJ
So from eqn 1
∆H = 8 45.54
= 364.32 kJ
Similarly from eqn
∆H = 68.91 1
= 68.91 kJ
Total ∆H = 364.32+68.91
Therefore, enthalpy change per mole of C2H5OH used 364.32+68.91/9
= 48.14 kJ
The enthalpy of neutralisation of HS- (aq) is - 5.1 kJ mol-1. Thus, second ionisation energy of H2S is- a)+52.6 kJ mol-1
- b) -13.7 kJ mol-1
- c)-52.2 kJ mol-1
- d) +52.2 kJ mol-1
Correct answer is option 'D'. Can you explain this answer?
The enthalpy of neutralisation of HS- (aq) is - 5.1 kJ mol-1. Thus, second ionisation energy of H2S is
a)
+52.6 kJ mol-1
b)
-13.7 kJ mol-1
c)
-52.2 kJ mol-1
d)
+52.2 kJ mol-1
|
|
Geetika Shah answered |
∆Hneutralisation = ∆HH+ + OH- + ∆rHionisation
-5.1 = -57.3 + ∆Hionisation
∆Hionisation = 52.2 kJ mol-1
-5.1 = -57.3 + ∆Hionisation
∆Hionisation = 52.2 kJ mol-1
The enthalpies of fusion for all reactions are- a)Negative
- b)Depends on the type of substance
- c)Zero
- d)Positive
Correct answer is option 'D'. Can you explain this answer?
The enthalpies of fusion for all reactions are
a)
Negative
b)
Depends on the type of substance
c)
Zero
d)
Positive
|
|
Lavanya Menon answered |
The enthalpies of fusion for all reactions are always positive because melting of a solid is an endothermic process in which heat is supplied to the reaction Hence the sum of enthalpies of the product is greater than the sum of enthalpies of the reactant.
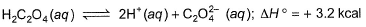 Thus, heat of neutralisation of H2C2O4 (aq) is
Thus, heat of neutralisation of H2C2O4 (aq) is- a)-16.9 kcal mol-1
- b)-10.5 kcal mol-1
- c)-24.2 kcal mol-1
- d) -30.6 kcal mol-1
Correct answer is option 'C'. Can you explain this answer?
Thus, heat of neutralisation of H2C2O4 (aq) is
a)
-16.9 kcal mol-1
b)
-10.5 kcal mol-1
c)
-24.2 kcal mol-1
d)
-30.6 kcal mol-1
|
|
Geetika Shah answered |
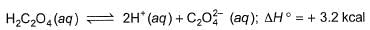
We can say that enthalpy of neutralisation of H2C2O4 means that we have 2 moles of H+. So, for weak acid, enthalpy of neutralisation = heat released in neutralisation of H+ and OH- + enthalpy of ionisation.
∆Hneutralisation = 2 (-13.6) + 3.2 = -24.2 kcal mol-1
Based on the following thermochemical reactions at 298 K and 1 bar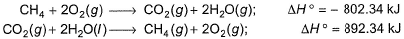 Q. Enthalpy of vaporisation of H2O (l) is
Q. Enthalpy of vaporisation of H2O (l) is - a)90 kJ mol-1
- b)45 kJ mol-1
- c)-90 kJ mol-1
- d)-45 kJ mol-1
Correct answer is option 'B'. Can you explain this answer?
Based on the following thermochemical reactions at 298 K and 1 bar
Q. Enthalpy of vaporisation of H2O (l) is
a)
90 kJ mol-1
b)
45 kJ mol-1
c)
-90 kJ mol-1
d)
-45 kJ mol-1
|
|
Gaurav Kumar answered |
Enthalpy of vaporization is defined for a substance when one mole of the substance is converted into the gaseous state. To obtain the enthalpy of vaporization of H2O liquid we will add reaction 1 and reaction 2 and then divide the enthalpy of x to since we need to calculate the enthalpy of vaporization for single mole of H2O
Latent heat of fusion of ice is 6.02 kJ mol-1. The heat capacity of water is 4.18 Jg-1K-1. 500 g of liquid water is to be cooled from 20°C to 0°C . Number of ice cubes (each of one mole) required is- a)1
- b)7
- c)14
- d)125
Correct answer is option 'B'. Can you explain this answer?
Latent heat of fusion of ice is 6.02 kJ mol-1. The heat capacity of water is 4.18 Jg-1K-1. 500 g of liquid water is to be cooled from 20°C to 0°C . Number of ice cubes (each of one mole) required is
a)
1
b)
7
c)
14
d)
125
|
|
Rajat Patel answered |
Molecular mass of hydrogen = 1
Molecular mass of oxygen =16
Molecular mass of water = 18 gram per mole
As,
n = m / M
n = 500 / 18
n = 27.8 moles
For cooling 27.8 moles at 20 = 27.8 x 75.4 x 20 = 41.922 kilo joule
41.922/6 = 6.987 that is approximately equal to 7
So, the number of ice cubes that are required to cool the water will be 7.
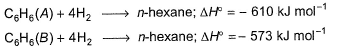
Given bond dissociation energy under standard states.
 Q. ii. Number of
Q. ii. Number of 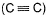 and
and 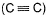 ) bond in (B) is .......
) bond in (B) is .......
Correct answer is '3'. Can you explain this answer?
Given bond dissociation energy under standard states.
Q. ii. Number of 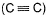 and
and 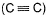 ) bond in (B) is .......
) bond in (B) is .......
|
|
Gaurav Kumar answered |
Number of bonds between C and C are 3
So the correct answer is 3.
So the correct answer is 3.
Enthalpy of neutralisation of a weak dibasic acid by NaOH is - 24.0 kcal. Thus, enthalpy of ionisation of dibasic acid is- a)10.3 kcal mol-1
- b)3.4 kcal mol-1
- c)13.7 kcal mol-1
- d)-13.7 kcal mol-1
Correct answer is option 'B'. Can you explain this answer?
Enthalpy of neutralisation of a weak dibasic acid by NaOH is - 24.0 kcal. Thus, enthalpy of ionisation of dibasic acid is
a)
10.3 kcal mol-1
b)
3.4 kcal mol-1
c)
13.7 kcal mol-1
d)
-13.7 kcal mol-1
|
|
Rajesh Gupta answered |
Enthalpy of neutralisation = ∆HH+ + OH- → H2O + ∆Hionisation
-24.0 = -27.4 + ∆Hionisation (since we have 2 moles of H+ and OH-, so enthalpy will also double)
∆Hionisation = 3.4 kcal mol-1
-24.0 = -27.4 + ∆Hionisation (since we have 2 moles of H+ and OH-, so enthalpy will also double)
∆Hionisation = 3.4 kcal mol-1
A rather soft, silvery metal was observed to have a specific heat of 0.225 cal K-1g-1. Thus, metal is- a)Na
- b)Mg
- c)Al
- d)Fe
Correct answer is option 'C'. Can you explain this answer?
A rather soft, silvery metal was observed to have a specific heat of 0.225 cal K-1g-1. Thus, metal is
a)
Na
b)
Mg
c)
Al
d)
Fe
|
|
Preeti Iyer answered |
It should be better to remove this question. It's data based.
Given, CH3COOH(aq) → CH3COO- (aq) + H+ (aq), ΔrH° = 0.005 kcal g-1Enthalpy change when 1 mole of Ca(OH)2, a strong base, is completely neutralised by CH3COOH (aq) in dilute solution is- a)-27.4 kcal mol-1
- b)-13.4 kcal mol-1
- c)-26.8 kcal mol-1
- d)-27.1 kcal mol-1
Correct answer is option 'C'. Can you explain this answer?
Given, CH3COOH(aq) → CH3COO- (aq) + H+ (aq), ΔrH° = 0.005 kcal g-1
Enthalpy change when 1 mole of Ca(OH)2, a strong base, is completely neutralised by CH3COOH (aq) in dilute solution is
a)
-27.4 kcal mol-1
b)
-13.4 kcal mol-1
c)
-26.8 kcal mol-1
d)
-27.1 kcal mol-1
|
|
It's Harmone answered |
Sahi jabab dea hai
The solubility of fluorides is much less as compared to corresponding chlorides- a)because of low lattice enthalpy.
- b)because of high lattice enthalpy.
- c)because of low ionization energy.
- d)because of high ionization energy.
Correct answer is option 'B'. Can you explain this answer?
The solubility of fluorides is much less as compared to corresponding chlorides
a)
because of low lattice enthalpy.
b)
because of high lattice enthalpy.
c)
because of low ionization energy.
d)
because of high ionization energy.
|
|
Om Desai answered |
Due to high lattice enthalpy of fluorides, it is difficult to break the lattice of fluoride compounds(due to the small size of fluorine, so the lattice is strong). SO the solubility of fluorides is less than chlorides.
Calculate resonance energy of N2O from the following dataΔfH° (N2O) = 82 kJ mol-1
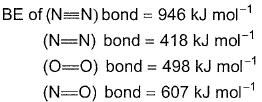
- a)-358 kJ mol-1
- b)170 kJ mol-1
- c)-88 kJ mol-1
- d)-170 kJ mol-1
Correct answer is option 'C'. Can you explain this answer?
Calculate resonance energy of N2O from the following data
ΔfH° (N2O) = 82 kJ mol-1
a)
-358 kJ mol-1
b)
170 kJ mol-1
c)
-88 kJ mol-1
d)
-170 kJ mol-1

|
Knowledge Hub answered |
= 946 + 249 - 607 - 418 = 1195 - 1025 = 170 kJ mol-1
Resonance energy = Observed heat of formation – calculated heat of formation
= 82 -170 = 88 kJ mol-1
Resonance energy = Observed heat of formation – calculated heat of formation
= 82 -170 = 88 kJ mol-1
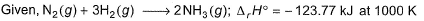 Thus,
Thus,- a)ΔfH° is - 61.885 kJ mol-1 at 1000 K
- b)ΔfE° is - 53.571 kJ mol-1 at 1000 K
- c)ΔfH° is - 44.42 kJ mol-1 at 300 K
- d)ΔfE° is - 41.93 kJ mol-1 at 300 K
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Thus,
a)
ΔfH° is - 61.885 kJ mol-1 at 1000 K
b)
ΔfE° is - 53.571 kJ mol-1 at 1000 K
c)
ΔfH° is - 44.42 kJ mol-1 at 300 K
d)
ΔfE° is - 41.93 kJ mol-1 at 300 K
|
|
Krishna Iyer answered |
The correct answer is option C
From, Kirchhoff's equation
△H2 (1000 K) = △H1(300 K) + △Cp(1000−300)
Here, △H2(1000 K) = −123.77 kJ mol−1
△H1(300 K)=?
△Cp = 2Cp(NH3) − [Cp(N2) + 3Cp(H2)]
=−6R
=−6 × 8.314 × 10−3kJ
∴−123.77 = △H1(300 K) + 6 × 8.314 × 10−3× 700
or △H1(300 K) = −88.85 kJ
For two moles of NH3
∴△Hf (NH3) = △H1(300K)/2
=−88.85/2
=−44.42 kJ mol−1
From, Kirchhoff's equation
△H2 (1000 K) = △H1(300 K) + △Cp(1000−300)
Here, △H2(1000 K) = −123.77 kJ mol−1
△H1(300 K)=?
△Cp = 2Cp(NH3) − [Cp(N2) + 3Cp(H2)]
=−6R
=−6 × 8.314 × 10−3kJ
∴−123.77 = △H1(300 K) + 6 × 8.314 × 10−3× 700
or △H1(300 K) = −88.85 kJ
For two moles of NH3
∴△Hf (NH3) = △H1(300K)/2
=−88.85/2
=−44.42 kJ mol−1
Direction (Q. Nos. 16 and 17) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).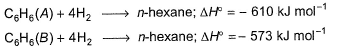
Given bond dissociation energy under standard states.
 Q. i. Number of
Q. i. Number of 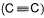 and
and 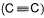 bonds in (A) is ......
bonds in (A) is ......
Correct answer is '2'. Can you explain this answer?
Direction (Q. Nos. 16 and 17) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Given bond dissociation energy under standard states.
Q. i. Number of  and
and 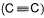 bonds in (A) is ......
bonds in (A) is ......
|
|
Suresh Reddy answered |
Let us assume the structures as given below and calculate bond energies,
Let us assume the given structures of C6H6 are:
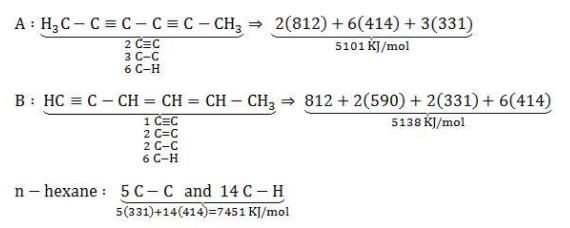
A + 4H2 ---> n - hexane
H =5101+4(435) -7451 = -610KJ/mol
B+4H2 ---> n - hexane
H =5138+4(435)-74551 =-573KJ/mol
Hence our assumed structures are correct.
Let us assume the given structures of C6H6 are:
A + 4H2 ---> n - hexane
H =5101+4(435) -7451 = -610KJ/mol
B+4H2 ---> n - hexane
H =5138+4(435)-74551 =-573KJ/mol
Hence our assumed structures are correct.
The increasing order of enthalpy of vaporization of NH3, PH3, and AsH3 is- a)NH3, AsH3, PH3
- b)NH3, PH3, AsH3
- c)AsH3, PH3, NH3
- d)PH3, AsH3, NH3
Correct answer is option 'D'. Can you explain this answer?
The increasing order of enthalpy of vaporization of NH3, PH3, and AsH3 is
a)
NH3, AsH3, PH3
b)
NH3, PH3, AsH3
c)
AsH3, PH3, NH3
d)
PH3, AsH3, NH3
|
|
Preeti Iyer answered |
The inter-particle forces are strongest in NH3 (Hydrogen bonds). In PH3 and AsH3 the inter-particle forces are dipole-dipole forces which are relatively stronger in AsH3. So the increasing order of vaporization is PH3, AsH3, and NH3 .
Enthalpies of formation of CO(g) , CO2 (g) , N2O (g) and N2O4 (g) are -110, - 393, 81 and 9.7 kJ mol-1. Thus, ΔrU for the reaction at 298 K is,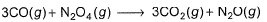
- a)+777.7 KJ
- b)- 777.7 KJ
- c)-211.7 KJ
- d)+211.7 KJ
Correct answer is option 'B'. Can you explain this answer?
Enthalpies of formation of CO(g) , CO2 (g) , N2O (g) and N2O4 (g) are -110, - 393, 81 and 9.7 kJ mol-1. Thus, ΔrU for the reaction at 298 K is,
a)
+777.7 KJ
b)
- 777.7 KJ
c)
-211.7 KJ
d)
+211.7 KJ
|
|
Preeti Iyer answered |
ΔrH=ΔrH(product)-ΔrH(reactant)
=3×-393+81+3×110-9.7
=-777.7kJ
ΔH = ΔU+ΔngRT
AS Δng = 0, ΔH = ΔU
So, ΔU = -777.7 kJ
=3×-393+81+3×110-9.7
=-777.7kJ
ΔH = ΔU+ΔngRT
AS Δng = 0, ΔH = ΔU
So, ΔU = -777.7 kJ
Given, BE of 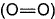 = 498.8 kJ mol-1
= 498.8 kJ mol-1
BE of (O—O) in ozone = 302.3 kJ mol-1Q. What is enthalpy change of the reaction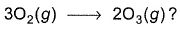
- a)286.8 kJ
- b)-286.8 kJ
- c)891.6 kJ
- d)445.8 kJ
Correct answer is option 'A'. Can you explain this answer?
Given, BE of 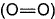 = 498.8 kJ mol-1
= 498.8 kJ mol-1
BE of (O—O) in ozone = 302.3 kJ mol-1
BE of (O—O) in ozone = 302.3 kJ mol-1
Q. What is enthalpy change of the reaction
a)
286.8 kJ
b)
-286.8 kJ
c)
891.6 kJ
d)
445.8 kJ
|
|
Lavanya Menon answered |
3O2(g) → 2O3(g)
∆H = 3(B.E)O2 - 2(B.E)O3
= 3(498.8) - 2(302.3)
= 287.2 kJ ml-1
∆H = 3(B.E)O2 - 2(B.E)O3
= 3(498.8) - 2(302.3)
= 287.2 kJ ml-1
Heat of neutralisation of a polybasic acid by a strong base is - 54.8 kcal mol-1? What is basicity of the acid?
Correct answer is '4'. Can you explain this answer?
Heat of neutralisation of a polybasic acid by a strong base is - 54.8 kcal mol-1? What is basicity of the acid?
|
|
Lavanya Menon answered |
For reaction of 1 mole of H+ and one mole of OH-, -13.6 kcal energy is released. As we have energy released = -54.8,
Therefore -13.6×nH+ = -54.8
nH+ = 4
So, the basicity of acid given here is 4.
Therefore -13.6×nH+ = -54.8
nH+ = 4
So, the basicity of acid given here is 4.
In a constant volume calorimeter, 3.5 g of a gas (molar mass = 28 g mol-1) was burnt in excess oxygen at 298.0 K. The temperature of the calorimeter was found to increase from 298.0 K to 298.45 K due to oxidation of the gas. Given that the heat capacity of the calorimeter is 2.5 kJ K-1, the numerical value of the heat given out in kJ mol-1 is[IIT JEE 2009]
Correct answer is '9'. Can you explain this answer?
In a constant volume calorimeter, 3.5 g of a gas (molar mass = 28 g mol-1) was burnt in excess oxygen at 298.0 K. The temperature of the calorimeter was found to increase from 298.0 K to 298.45 K due to oxidation of the gas. Given that the heat capacity of the calorimeter is 2.5 kJ K-1, the numerical value of the heat given out in kJ mol-1 is
[IIT JEE 2009]
|
|
Lavanya Menon answered |
Energy released by combustion of 3.5 g gas = 2.5×(298.45-298) kJ
Energy released by 1 mole of gas = 2.5×0.45/3.5/28 = 9 kJ mol-1
Energy released by 1 mole of gas = 2.5×0.45/3.5/28 = 9 kJ mol-1
Given, BE (H—H) - x1, BE 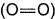 = x2, BE (O—H) = x3 and for H2O (l) → H2O (g), ΔH = x4 mol-1, then ΔfH° [H2O (l)] is
= x2, BE (O—H) = x3 and for H2O (l) → H2O (g), ΔH = x4 mol-1, then ΔfH° [H2O (l)] is- a)
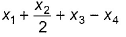
- b)
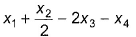
- c)
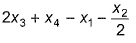
- d)2x1 + x2 - 4x3 - 2x4
Correct answer is option 'B'. Can you explain this answer?
Given, BE (H—H) - x1, BE 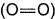 = x2, BE (O—H) = x3 and for H2O (l) → H2O (g), ΔH = x4 mol-1, then ΔfH° [H2O (l)] is
= x2, BE (O—H) = x3 and for H2O (l) → H2O (g), ΔH = x4 mol-1, then ΔfH° [H2O (l)] is
a)
b)
c)
d)
2x1 + x2 - 4x3 - 2x4
|
|
Gaurav Kumar answered |
Required reaction= Heat of formation of water – H2(g) +1/2O2 (g)= H2O(l)
WRITE THE EQUATION PROPERLY FOR EACH EQUATION
rearranging the reactions we have to get the above equation
B.E. of H--H= H2(g) → 2H(g)..........X1
B.E of O2 = O2(g) ---> 2O(g)...........X2
B.E. OF O-H = O-H(aq)--> O(g)+H(g) …......X3
LATENT HEAT OF VAPOURISATION OF WATER = H2O(l)----> H2O(g)..........X4
THUS REVERSING EQTN X3 AND X4 , HALVING EQUATION X2 AND DOUBLING EQUATION X3 WE WILL GET THE DESIRED EQUATION.
WRITE THE EQUATION PROPERLY FOR EACH EQUATION
rearranging the reactions we have to get the above equation
B.E. of H--H= H2(g) → 2H(g)..........X1
B.E of O2 = O2(g) ---> 2O(g)...........X2
B.E. OF O-H = O-H(aq)--> O(g)+H(g) …......X3
LATENT HEAT OF VAPOURISATION OF WATER = H2O(l)----> H2O(g)..........X4
THUS REVERSING EQTN X3 AND X4 , HALVING EQUATION X2 AND DOUBLING EQUATION X3 WE WILL GET THE DESIRED EQUATION.
The heat capacity of a calorimeter (commonly called the calorimeter constant) was determined by heating the calorimeter and its content using an electrical heater. If ΔT = 1.221 K as 1.25 A of electricity at 3.26 V was passed through the heater immersed in 137.5 g of water in the calorimeter for 175 s, determine the calorimeter constant (in JK-1). (Specific heat of water at constant pressure is 75.291 JK-1 mol-1).
Correct answer is '9'. Can you explain this answer?
The heat capacity of a calorimeter (commonly called the calorimeter constant) was determined by heating the calorimeter and its content using an electrical heater. If ΔT = 1.221 K as 1.25 A of electricity at 3.26 V was passed through the heater immersed in 137.5 g of water in the calorimeter for 175 s, determine the calorimeter constant (in JK-1). (Specific heat of water at constant pressure is 75.291 JK-1 mol-1).
|
|
Poulomi Singh answered |
Subtracting the energy gained by the cold water from the energy lost by the hot water. This will give us the amount of energy gained by the calorimeter. Dividing the energy gained by the calorimeter by Tc (the temperature change of the cold water). This final answer is calorimeter constant
ms(dT)=137.5×4.184×1.221=702
joules=coulomb×volt =. 1.25×175×3.26=713
713 - 702 = 11
(11/1.221) = 9
ms(dT)=137.5×4.184×1.221=702
joules=coulomb×volt =. 1.25×175×3.26=713
713 - 702 = 11
(11/1.221) = 9
Given the following thermochemical data at 298 K and 1 barΔH°vap (CH3OH) = 38.0 kJ mol-1
ΔfH°: H(g) = 218 kJ mol-1
O(g) = 249 kJ mol-1
C(g) = 715 kJ mol-1Bond dissociation energy(C—H) = 415 kJ mol-1
(C—O) = 356 kJ mol-1
(O—H) = 4 6 3 kJ mol-1Q. The ΔfH° of liquid methyl alcohol in kJ mol-1 is- a)-170 kJ mol-1
- b)+170 kJ mol-1
- c)-266 kJ mol-1
- d)+266 kJ mol-1
Correct answer is option 'D'. Can you explain this answer?
Given the following thermochemical data at 298 K and 1 bar
ΔH°vap (CH3OH) = 38.0 kJ mol-1
ΔfH°: H(g) = 218 kJ mol-1
O(g) = 249 kJ mol-1
C(g) = 715 kJ mol-1
ΔfH°: H(g) = 218 kJ mol-1
O(g) = 249 kJ mol-1
C(g) = 715 kJ mol-1
Bond dissociation energy
(C—H) = 415 kJ mol-1
(C—O) = 356 kJ mol-1
(O—H) = 4 6 3 kJ mol-1
(C—O) = 356 kJ mol-1
(O—H) = 4 6 3 kJ mol-1
Q. The ΔfH° of liquid methyl alcohol in kJ mol-1 is
a)
-170 kJ mol-1
b)
+170 kJ mol-1
c)
-266 kJ mol-1
d)
+266 kJ mol-1
|
|
Muhammed M.a answered |
Sorry first time see this question
Hydration energies of Li+ and Cl- ions are - 499 and - 382 kJ mol-1 respectively. If lattice energy of LiCI is - 840 kJ mol-1 then heat of solution of LiCI is - a)+41 kJ mol -1
- b)-41 kJ mol -1
- c)-1621 kJ mol -1
- d)-1222 kJ mol -1
Correct answer is option 'B'. Can you explain this answer?
Hydration energies of Li+ and Cl- ions are - 499 and - 382 kJ mol-1 respectively. If lattice energy of LiCI is - 840 kJ mol-1 then heat of solution of LiCI is
a)
+41 kJ mol -1
b)
-41 kJ mol -1
c)
-1621 kJ mol -1
d)
-1222 kJ mol -1
|
|
Ankita Menon answered |
Li+ + H2O → Li+(aq.)
∆H1 = -499 kJ mol-1 ---------- (I)
Cl- + H2O →Cl-(aq)
∆H2 = -382 kJ mol-1 ---------- (II)
Li+ + Cl- → LiCl(s)
∆H3 = -840 kJ mol-1---------- (III)
For r the heat of solution, we need the reaction,
LiCl(s) → Li+(aq.) + Cl-(aq)
This can be obtained by (I) + (II) - (III)
So the ∆H value be = -499 - 382 -(-840) = -41 kJ mol-1.
∆H1 = -499 kJ mol-1 ---------- (I)
Cl- + H2O →Cl-(aq)
∆H2 = -382 kJ mol-1 ---------- (II)
Li+ + Cl- → LiCl(s)
∆H3 = -840 kJ mol-1---------- (III)
For r the heat of solution, we need the reaction,
LiCl(s) → Li+(aq.) + Cl-(aq)
This can be obtained by (I) + (II) - (III)
So the ∆H value be = -499 - 382 -(-840) = -41 kJ mol-1.
Given, 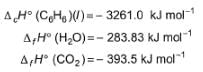 Q. Thus, ΔfH° of (C6H6) (l) is
Q. Thus, ΔfH° of (C6H6) (l) is- a)-3267.0 kJ mol-1
- b)-48.51 kJ mol-1
- c)+3267.0 kJ mol-1
- d)+48.51 kJ mol-1
Correct answer is option 'D'. Can you explain this answer?
Given,
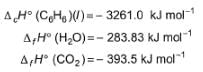
Q. Thus, ΔfH° of (C6H6) (l) is
a)
-3267.0 kJ mol-1
b)
-48.51 kJ mol-1
c)
+3267.0 kJ mol-1
d)
+48.51 kJ mol-1
|
|
Gaurav Kumar answered |
C6H6(l) + 15/2O2(g) → 6CO2(g) + 3H2O(l)
∆Hr = (∆Hc)C6H6 ⇒-3261 = ∑Hproduct - ∑Hreactant = 6(-393.5) -3(-283.83) - ∆fHC6H6
∆Hf = 48 kJ mol-1
∆Hr = (∆Hc)C6H6 ⇒-3261 = ∑Hproduct - ∑Hreactant = 6(-393.5) -3(-283.83) - ∆fHC6H6
∆Hf = 48 kJ mol-1
Direction (Q. Nos. 9) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.Q.Statement I : Cv value of helium (He) is always 3/2R but Cv value of hydrogen (H2) is 3/2R at low temperature, 5/2R at moderate temperature and more than 5/2R at higher temperature.Statement II : At lower temperature, only translational degree of freedom contributes to heat capacity while at higher temperature, rotational and vibrational degrees of freedom also contribute to heat capacity.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 9) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q.
Statement I : Cv value of helium (He) is always 3/2R but Cv value of hydrogen (H2) is 3/2R at low temperature, 5/2R at moderate temperature and more than 5/2R at higher temperature.
Statement II : At lower temperature, only translational degree of freedom contributes to heat capacity while at higher temperature, rotational and vibrational degrees of freedom also contribute to heat capacity.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Bhargavi Joshi answered |
In case of helium (monoatomic gas) we have only three degrees of freedom which correspond to three translational motion so the total heat capacity will increase. The contributors by vibrational motion are not appreciable at low temperature but increase from 0 to R when temperature increases.
CV=−f(R)/2
CV=-f(R)2, where f is the degree of freedom. At low temperature only translational motion is considered and f=3
∴CV=3R/2
f=3.∴CV=3R2 At moderate temperature both translational and rotational motions are considered.
f=3+2
f=3+2 (3-translational and 2 rotational).
∴CV=5R/2
∴CV=5R2. At still high temperature translational, rotational and vibrational motions are considered.
f=3+2+2
f=3+2+2 (3-translatinal , 2-rotational, 2-vibrational).
∴CV=7R/2
CV=−f(R)/2
CV=-f(R)2, where f is the degree of freedom. At low temperature only translational motion is considered and f=3
∴CV=3R/2
f=3.∴CV=3R2 At moderate temperature both translational and rotational motions are considered.
f=3+2
f=3+2 (3-translational and 2 rotational).
∴CV=5R/2
∴CV=5R2. At still high temperature translational, rotational and vibrational motions are considered.
f=3+2+2
f=3+2+2 (3-translatinal , 2-rotational, 2-vibrational).
∴CV=7R/2
Direction (Q. Nos. 16 - 18) This section contains 3 questions. when worked out will result in an integer from 0 to 9 (both inclusive).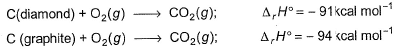 Q. How much heat (in kcal) is required to convert 36 g of diamond into graphite?
Q. How much heat (in kcal) is required to convert 36 g of diamond into graphite?
Correct answer is '9'. Can you explain this answer?
Direction (Q. Nos. 16 - 18) This section contains 3 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q. How much heat (in kcal) is required to convert 36 g of diamond into graphite?
|
|
Geetika Shah answered |
C(diamond) + O2(g) → CO2(g) -----(i) ∆rH° = -91 kcal mol-1
C(graphite) + O2(g) → CO2(g) -----(ii) ∆rH° = -94 kcal mol-1
For 36 gm of C(diamond), we have 3 moles of diamond,
On (i)-(ii), we get → C(graphite) ∆rH° =-91-( -94 ) kcal mol-1 = 3 kcal mol-1
So for 3 moles, we have 3×3 = 9 kcal
C(diamond)
C(graphite) + O2(g) → CO2(g) -----(ii) ∆rH° = -94 kcal mol-1
For 36 gm of C(diamond), we have 3 moles of diamond,
On (i)-(ii), we get → C(graphite) ∆rH° =-91-( -94 ) kcal mol-1 = 3 kcal mol-1
So for 3 moles, we have 3×3 = 9 kcal
C(diamond)
Direction (Q. Nos. 1-13) This section contains 13 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. If the enthalpy of HCI (g) and Cl- (aq) are - 92.30 kJ mol-1 and - 167.44 kJ mol-1 respectively, then ΔrH° for the following reaction isHCI (g) + aq → H+ (aq) + Cl- (aq)- a)-75.14 kJ mol-1
- b)+75.14 kJ mol-1
- c)-259.74 kJ mol-1
- d)0.00 kJ mol-1
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 1-13) This section contains 13 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. If the enthalpy of HCI (g) and Cl- (aq) are - 92.30 kJ mol-1 and - 167.44 kJ mol-1 respectively, then ΔrH° for the following reaction is
HCI (g) + aq → H+ (aq) + Cl- (aq)
a)
-75.14 kJ mol-1
b)
+75.14 kJ mol-1
c)
-259.74 kJ mol-1
d)
0.00 kJ mol-1
|
|
Om Desai answered |
Enthalpy of reaction = ∑enthalpy of products - ∑enthalpy of reactants
Therefore
∆Hreaction = -167.44 - (- 92.30) (∆Hformation of H+ is 0)
= -75.14 kilo J / mole
Therefore
∆Hreaction = -167.44 - (- 92.30) (∆Hformation of H+ is 0)
= -75.14 kilo J / mole
In the following reactions,I. 30 mL of 0.1 M Ba(OH)2(aq) + 30 mL of 0.1 M H2SO4 (aq), rise intemperature = ΔT1
II. 90 mL of 0.1 M Ba(OH)2(aq) + 90 mL of 0.1 M H2SO4 (aq), rise intemperature = ΔT2. Thus,- a)ΔT2 = 3ΔT1
- b)ΔT1 = ΔT2
- c)ΔT1 = 3ΔT2
- d)ΔT1 = 2ΔT2
Correct answer is option 'A'. Can you explain this answer?
In the following reactions,
I. 30 mL of 0.1 M Ba(OH)2(aq) + 30 mL of 0.1 M H2SO4 (aq), rise intemperature = ΔT1
II. 90 mL of 0.1 M Ba(OH)2(aq) + 90 mL of 0.1 M H2SO4 (aq), rise intemperature = ΔT
II. 90 mL of 0.1 M Ba(OH)2(aq) + 90 mL of 0.1 M H2SO4 (aq), rise intemperature = ΔT
2
. Thus,a)
ΔT2 = 3ΔT1
b)
ΔT1 = ΔT2
c)
ΔT1 = 3ΔT2
d)
ΔT1 = 2ΔT2
|
|
Nayanika Chakraborty answered |
Solution:
Given:
30 mL of 0.1 M Ba(OH)2(aq) 30 mL of 0.1 M H2SO4(aq), rise in temperature = T1
90 mL of 0.1 M Ba(OH)2(aq) 90 mL of 0.1 M H2SO4(aq), rise in temperature = T2
To determine: T2 in terms of T1
Explanation:
The given reactions are acid-base neutralization reactions that liberate heat. The heat is measured in terms of a rise in temperature.
The heat liberated in a reaction is directly proportional to the number of moles of the limiting reagent. In the given reactions, the limiting reagent is H2SO4.
Let's calculate the number of moles of H2SO4 in both reactions.
In the first reaction, the volume of H2SO4 is 30 mL, which is the same as the volume of Ba(OH)2. Therefore, the number of moles of H2SO4 is:
n(H2SO4) = Molarity × Volume = 0.1 × 0.03 = 0.003 moles
In the second reaction, the volume of H2SO4 is three times the volume in the first reaction. Therefore, the number of moles of H2SO4 is:
n(H2SO4) = Molarity × Volume = 0.1 × 0.09 = 0.009 moles
As the number of moles of H2SO4 is three times greater in the second reaction, the heat liberated will also be three times greater.
Therefore, T2 = 3T1
Hence, option A is the correct answer.
Given:
30 mL of 0.1 M Ba(OH)2(aq) 30 mL of 0.1 M H2SO4(aq), rise in temperature = T1
90 mL of 0.1 M Ba(OH)2(aq) 90 mL of 0.1 M H2SO4(aq), rise in temperature = T2
To determine: T2 in terms of T1
Explanation:
The given reactions are acid-base neutralization reactions that liberate heat. The heat is measured in terms of a rise in temperature.
The heat liberated in a reaction is directly proportional to the number of moles of the limiting reagent. In the given reactions, the limiting reagent is H2SO4.
Let's calculate the number of moles of H2SO4 in both reactions.
In the first reaction, the volume of H2SO4 is 30 mL, which is the same as the volume of Ba(OH)2. Therefore, the number of moles of H2SO4 is:
n(H2SO4) = Molarity × Volume = 0.1 × 0.03 = 0.003 moles
In the second reaction, the volume of H2SO4 is three times the volume in the first reaction. Therefore, the number of moles of H2SO4 is:
n(H2SO4) = Molarity × Volume = 0.1 × 0.09 = 0.009 moles
As the number of moles of H2SO4 is three times greater in the second reaction, the heat liberated will also be three times greater.
Therefore, T2 = 3T1
Hence, option A is the correct answer.
Given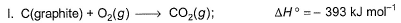
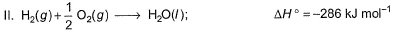
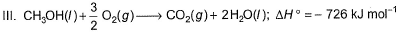 Q. Thus, heat of formation of CH3OH(/)is
Q. Thus, heat of formation of CH3OH(/)is- a)- 239.0 kJ mol-1
- b)+ 239.0 kJ mol-1
- c)- 47.0 kJ mol-1
- d)+ 47.0 kJ mol-1
Correct answer is option 'A'. Can you explain this answer?
Given
Q. Thus, heat of formation of CH3OH(/)is
a)
- 239.0 kJ mol-1
b)
+ 239.0 kJ mol-1
c)
- 47.0 kJ mol-1
d)
+ 47.0 kJ mol-1
|
|
Neha Joshi answered |
Let's number our eqns-
CH3OH + 3/2 O2 → CO2 + 2H2O ...(1)
ΔH1 = –726 kJ/mol
C + O2 → CO2 ...(2)
ΔH2 = –393 kJ/mol
H2 + 1/2 O2 → H2O ...(3)
ΔH3 = –286 kJ/mol
Eqn(2) + 2×eqn(3) - eqn(1) :-
C + O2 + 2H2 + O2 + CO2 + 2H20→ CO2 + 2H20 + CH3OH + 3/2 O2
C + 1/2 O2 + 2H2 → CH3OH
Thus enthalpy of formation-
∆Hf(CH3OH) = ∆H2 + 2∆H3 - ∆H1
∆Hf(CH3OH) = -393 + 2(-286) + 726
∆Hf(CH3OH) = -239 kJ/mol
CH3OH + 3/2 O2 → CO2 + 2H2O ...(1)
ΔH1 = –726 kJ/mol
C + O2 → CO2 ...(2)
ΔH2 = –393 kJ/mol
H2 + 1/2 O2 → H2O ...(3)
ΔH3 = –286 kJ/mol
Eqn(2) + 2×eqn(3) - eqn(1) :-
C + O2 + 2H2 + O2 + CO2 + 2H20→ CO2 + 2H20 + CH3OH + 3/2 O2
C + 1/2 O2 + 2H2 → CH3OH
Thus enthalpy of formation-
∆Hf(CH3OH) = ∆H2 + 2∆H3 - ∆H1
∆Hf(CH3OH) = -393 + 2(-286) + 726
∆Hf(CH3OH) = -239 kJ/mol
Chapter doubts & questions for Enthalpy - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Enthalpy - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily