All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Aromatic Compounds for EmSAT Achieve Exam
Organic compounds can be classified even based upon the function groups. Identify the one which is not a functional group
- a)Isocyanide
- b)Isocyano
- c)Carboxyl
- d)Carbonyl
Correct answer is option 'A'. Can you explain this answer?
Organic compounds can be classified even based upon the function groups. Identify the one which is not a functional group
a)
Isocyanide
b)
Isocyano
c)
Carboxyl
d)
Carbonyl
|
|
Krishna Iyer answered |
Isocyanide is a compound and it is not a functional group.
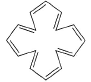
What is true about the compound calicene?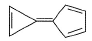
- a)It is highly soluble in water
- b)It exists mainly as dipolar ion with +ve charge in the three membered ring while -ve charge in five membered ring
- c)It exists mainly as dipolar ion with -ve charge in the three membered ring and +ve charge in five membered ring
- d)In solution it shows very high electrical conductivity
Correct answer is option 'A,B,D'. Can you explain this answer?
What is true about the compound calicene?
a)
It is highly soluble in water
b)
It exists mainly as dipolar ion with +ve charge in the three membered ring while -ve charge in five membered ring
c)
It exists mainly as dipolar ion with -ve charge in the three membered ring and +ve charge in five membered ring
d)
In solution it shows very high electrical conductivity
|
|
Raghav Bansal answered |
- In calicene, the electrons move towared the five-membered ring because both rings are aromatic in the resonance contributor that has a negative charge on a carbon of the five-membered ring and a positive charge on a carbon of the three-membered ring
- It is highly soluble in water and in solution it shows very high electrical conductivity
So, Option C is not correct and other Options are Correct.
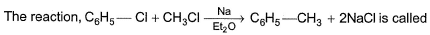
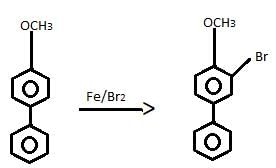
- a)Fittig reaction
- b)Wurtz’s-Fittig reaction
- c)Ullmann reaction
- d)Wurtz’s reaction
Correct answer is option 'B'. Can you explain this answer?
a)
Fittig reaction
b)
Wurtz’s-Fittig reaction
c)
Ullmann reaction
d)
Wurtz’s reaction
|
|
Om Desai answered |
The correct answer is option B
Wurtz - Fittig reaction:
Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene.
For example, bromobenzene reacts with methyl bromide in presence of sodium. dry ether to form toluene.
C6H6 - Br + CH3 - Br + 2Na(dry ether)------> C6H5 - CH3 + 2NaBr
Wurtz - Fittig reaction:
Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene.
For example, bromobenzene reacts with methyl bromide in presence of sodium. dry ether to form toluene.
C6H6 - Br + CH3 - Br + 2Na(dry ether)------> C6H5 - CH3 + 2NaBr
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Arrange the following compounds in increasing order of polarity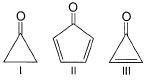
- a)I < II < III
- b)III < II < I
- c)II < I < III
- d)III < I < II
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Arrange the following compounds in increasing order of polarity
a)
I < II < III
b)
III < II < I
c)
II < I < III
d)
III < I < II
|
|
Om Desai answered |
In case 1, the bond is broken in oxygen’s favor and it will attain its octet. Also, carbon becomes sp2 hybridized, so there is a chance of polarity.
In case 2, if the bond is broken in favor of oxygen, then the ring will become anti-aromatic which is highly unstable and the bond won’t be broken in that way. If the bond is broken in favor of carbon in the ring, then although the ring becomes aromatic but oxygen will bear +ve charge which is very unstable. So, there is no chance to break the bond.
In case 3, if the double bond is broken in favor of oxygen, then oxygen will acquire a negative charge and the ring will become aromatic. So, it is a highly favorable case of double bond breaking.
Therefore, the order of polarity: - III>I>II
In case 2, if the bond is broken in favor of oxygen, then the ring will become anti-aromatic which is highly unstable and the bond won’t be broken in that way. If the bond is broken in favor of carbon in the ring, then although the ring becomes aromatic but oxygen will bear +ve charge which is very unstable. So, there is no chance to break the bond.
In case 3, if the double bond is broken in favor of oxygen, then oxygen will acquire a negative charge and the ring will become aromatic. So, it is a highly favorable case of double bond breaking.
Therefore, the order of polarity: - III>I>II
How many monobromo derivatives exists for anthracene?
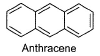
- a)2
- b)3
- c)4
- d)5
Correct answer is option 'B'. Can you explain this answer?
How many monobromo derivatives exists for anthracene?
a)
2
b)
3
c)
4
d)
5
|
|
Suresh Iyer answered |
The correct answer is Option B.
There are 3 monobromo derivatives exists for anthracene:
1-Chloroanthracene
2-Chloroanthracene
and 9-Chloroanthracene
1-Chloroanthracene
2-Chloroanthracene
and 9-Chloroanthracene
Arrange the halogens F2, Cl2, Br2, I2 in order of their increasing reactivity with alkanes.- a)I2 < Br2 < Cl2 < F2
- b)Br2 < Cl2 < F2 < I2
- c)F2 < Cl2 < Br2 < I2
- d)Br2 < I2 < Cl2 < F2
Correct answer is option 'A'. Can you explain this answer?
Arrange the halogens F2, Cl2, Br2, I2 in order of their increasing reactivity with alkanes.
a)
I2 < Br2 < Cl2 < F2
b)
Br2 < Cl2 < F2 < I2
c)
F2 < Cl2 < Br2 < I2
d)
Br2 < I2 < Cl2 < F2
|
|
Gaurav Kumar answered |
The correct answer is Option A.
Since reactivity decreases down the group as the electronegativity of the halogen decreases down the group. Thus, rate of reaction of alkanes with halogens is
I2 < Br2 < Cl2 <F2
Since reactivity decreases down the group as the electronegativity of the halogen decreases down the group. Thus, rate of reaction of alkanes with halogens is
I2 < Br2 < Cl2 <F2
Consider the following bromides :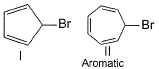
- a)Both decomposes to form
- b)Both decomposes to forms Br+
- c)I decomposes to give
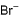 and II gives Br+
and II gives Br+ - d)I decomposes to give Br+ while II gives
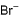
Correct answer is option 'D'. Can you explain this answer?
Consider the following bromides :
a)
Both decomposes to form 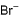
b)
Both decomposes to forms Br+
c)
I decomposes to give 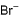 and II gives Br+
and II gives Br+
d)
I decomposes to give Br+ while II gives 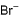
|
|
Geetika Shah answered |
In the compound I, Br will dispatch as Br+ so that a -ve charge appears on carbon which will give us 6π electrons. So ring will become aromatic.(4n+2 π electron is needed for aromaticity)
In compound II, Br will dispatch as Br-. So that carbon has +ve charge and all the double bond will circulate in the ring. This will maintain 4n+2 π electron and the molecule will remain as aromatic.
In compound II, Br will dispatch as Br-. So that carbon has +ve charge and all the double bond will circulate in the ring. This will maintain 4n+2 π electron and the molecule will remain as aromatic.
Select the species which is not aromatic.- a)
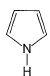
- b)
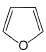
- c)
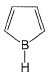
- d)
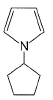
Correct answer is option 'C'. Can you explain this answer?
Select the species which is not aromatic.
a)
b)
c)
d)
|
|
Gaurav Kumar answered |
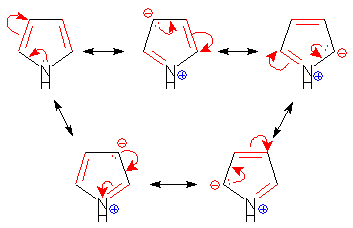
Here, we can see that Nitrogen gave its lone pair to make the system aromatic. The same case happens with option b and d. But with option c, Boron is not having any lone pair to donate. So option c is correct answer
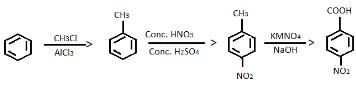
Provide the appropriate sequence of reagents that can bring about the following transformation.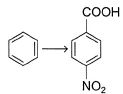
- a)CH3CI/AICI3 then conc.HNO3/conc.H2SO4 then KMnO4/NaOH
- b)cone. HNO3 /conc.H2SO4 then CH3CI/AICI3 then KMnO4/NaOH
- c)CH3CI/AICI3 then KMnO4/NaOH then conc.HNO3/conc.H2SO4
- d)conc.H2SO4 /conc.HNO3 then CH3COCI/AICI3 then KMnO4/NaOH
Correct answer is option 'A'. Can you explain this answer?
Provide the appropriate sequence of reagents that can bring about the following transformation.
a)
CH3CI/AICI3 then conc.HNO3/conc.H2SO4 then KMnO4/NaOH
b)
cone. HNO3 /conc.H2SO4 then CH3CI/AICI3 then KMnO4/NaOH
c)
CH3CI/AICI3 then KMnO4/NaOH then conc.HNO3/conc.H2SO4
d)
conc.H2SO4 /conc.HNO3 then CH3COCI/AICI3 then KMnO4/NaOH
|
|
Naina Sharma answered |
The correct answer is Option A.

What is major product of the reaction below?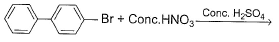
- a)
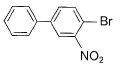
- b)
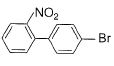
- c)
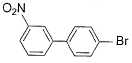
- d)
Correct answer is option 'D'. Can you explain this answer?
What is major product of the reaction below?
a)
b)
c)
d)
|
|
Lavanya Menon answered |
The correct answer is option D


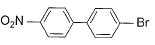
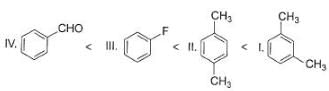
What is the correct order of increasing acidic strength of the following?
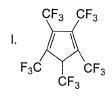
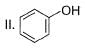
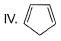
- a)Ill < I < IV < II
- b)Ill < IV < II < I
- c) Ill < II < IV < I
- d)III<IV<I<II
Correct answer is option 'B'. Can you explain this answer?
What is the correct order of increasing acidic strength of the following?




a)
Ill < I < IV < II
b)
Ill < IV < II < I
c)
Ill < II < IV < I
d)
III<IV<I<II
|
|
Lavanya Menon answered |
Compound I is having the highest acidic strength due to the -I effect of five CF3 substituents.
Compound II is having less acidic strength than I but more than the rest due to the extremely stable conjugate anion formed after deprrotonation.
So, Option B is correct.
When two or more different substituents are attached with a benzene ring the number 1 position in the ring is given to a high priority group. Which one of the following groups has highest - priority?- a)?NH2
- b)?CHO
- c)?COOH
- d)?CN
Correct answer is option 'C'. Can you explain this answer?
When two or more different substituents are attached with a benzene ring the number 1 position in the ring is given to a high priority group. Which one of the following groups has highest - priority?
a)
?NH2
b)
?CHO
c)
?COOH
d)
?CN
|
|
Raghav Bansal answered |
Option C is correct, because in Nomenclature of Organic Compounds. Priority is given to that functional group that is on the top of the Priority Order of Functional Groups. Among the given Functional Groups COOH is having the highest priority so, the number 1 position in the ring is given to it.
In principle, what is true regarding benzene and 1, 3, 5-cyclohexatriene?- a)Theoretically they are the same molecules
- b)Both have same lengths of all of their C — C bonds
- c)Both have same enthalpy of hydrogenation
- d)Cyclohexatriene has two different C — C bond lengths while benzene has only one type of C — C bond length whose value is between those of cyclohexatriene
Correct answer is option 'D'. Can you explain this answer?
In principle, what is true regarding benzene and 1, 3, 5-cyclohexatriene?
a)
Theoretically they are the same molecules
b)
Both have same lengths of all of their C — C bonds
c)
Both have same enthalpy of hydrogenation
d)
Cyclohexatriene has two different C — C bond lengths while benzene has only one type of C — C bond length whose value is between those of cyclohexatriene
|
|
Lavanya Menon answered |
The correct answer is Option D.
In 1,3,5-cyclohexatriene, there are three C = C having a bond length 134 pm and three C - C having a bond length 154 pm. In benzene, all the six C - C bonds have the same bond length 139 pm.
In 1,3,5-cyclohexatriene, there are three C = C having a bond length 134 pm and three C - C having a bond length 154 pm. In benzene, all the six C - C bonds have the same bond length 139 pm.
Statement I : The follow compound is optically active.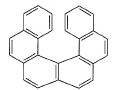 Statement II : It has a chiral carbon.
Statement II : It has a chiral carbon.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement I is incorrect but Statement II is correct
Correct answer is option 'C'. Can you explain this answer?
Statement I : The follow compound is optically active.
Statement II : It has a chiral carbon.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement I is incorrect but Statement II is correct

|
Pioneer Academy answered |
The correct answer is option C
Statement 1 is correct because Plane Of Symmetry and Centre Of Symmetry are absent in the compound .
Statement 2 is clearly incorrect as there is No 'Sp3 ' Hybridized Carbon atom .
Statement 2 is clearly incorrect as there is No 'Sp3 ' Hybridized Carbon atom .
What is true about sulphonation of C6H6?- a)Cone. H2SO4 is boiling condition brings about sulphonation
- b)SO3 is electrophile in sulphonation reaction
- c)Sulphonation reaction is reversible in nature
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
What is true about sulphonation of C6H6?
a)
Cone. H2SO4 is boiling condition brings about sulphonation
b)
SO3 is electrophile in sulphonation reaction
c)
Sulphonation reaction is reversible in nature
d)
All of the above

|
Ashish Mishra answered |
D is correct.
Which of the following systems are aromatic?
- a)
- b)
- c)

- d)
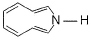
Correct answer is option 'C,D'. Can you explain this answer?
Which of the following systems are aromatic?
a)
b)
c)
d)
|
|
Pooja Shah answered |
The correct answers are Options C and D.
Aromatic compounds are those which follow Huckel's rule i.e, they have (4n+2) π electrons, n must be an integer.
In option C, there are 5 π bonds which means 10 π electrons; so 4n+2 = 10 i.e, n= 2 which is an integer.
In option D, Nitrogen has a lone pair which contains 2 electrons therefore this compound also have 10 π electrons; so n= 2.
Aromatic compounds are those which follow Huckel's rule i.e, they have (4n+2) π electrons, n must be an integer.
In option C, there are 5 π bonds which means 10 π electrons; so 4n+2 = 10 i.e, n= 2 which is an integer.
In option D, Nitrogen has a lone pair which contains 2 electrons therefore this compound also have 10 π electrons; so n= 2.
Direction (Q. No. 22 - 25) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q. C6H4Br2(P) is a dibromo benzene and it is the less polar isomer of its two polar isomers. If P is treated with conc. HNO3 and conc -H2SO4 mixture, in principle, how many mono nitration products are expected?
Correct answer is '2'. Can you explain this answer?
Direction (Q. No. 22 - 25) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q. C6H4Br2(P) is a dibromo benzene and it is the less polar isomer of its two polar isomers. If P is treated with conc. HNO3 and conc -H2SO4 mixture, in principle, how many mono nitration products are expected?

|
Stepway Academy answered |
Understanding the Compound
- The compound C6H4Br2(P) is a dibromo benzene. This means it has two bromine atoms attached to the benzene ring.
Understanding Isomerism
- The less polar isomer refers to the geometric arrangement of the bromine atoms on the benzene ring that results in less polarity.
- In the case of dibromo benzene, there are three possible geometric arrangements (ortho, meta, and para) but only ortho-dibromo benzene and para-dibromo benzene are polar. The less polar of the two is the para isomer.
Nitration Reaction
- Nitration is a general class of a chemical process for the introduction of a nitro group into an organic chemical compound.
- The nitration of benzene involves substitution of a hydrogen atom by a nitro group (-NO2) and this is facilitated by a mixture of concentrated nitric acid (HNO3) and sulfuric acid (H2SO4).
Mono Nitration Products
- Considering that the compound is para-dibromo benzene, the nitration can occur at any of the 4 remaining carbon atoms in the benzene ring.
- However, since the question asks for mono nitration products (meaning only one nitro group is added), the number of unique products will depend on the symmetry of the molecule.
- For para-dibromo benzene, the molecule is symmetric. Nitration at any of the 4 remaining positions will result in only 2 unique mono-nitration products due to this symmetry.
So, the answer is 2.
- The compound C6H4Br2(P) is a dibromo benzene. This means it has two bromine atoms attached to the benzene ring.
Understanding Isomerism
- The less polar isomer refers to the geometric arrangement of the bromine atoms on the benzene ring that results in less polarity.
- In the case of dibromo benzene, there are three possible geometric arrangements (ortho, meta, and para) but only ortho-dibromo benzene and para-dibromo benzene are polar. The less polar of the two is the para isomer.
Nitration Reaction
- Nitration is a general class of a chemical process for the introduction of a nitro group into an organic chemical compound.
- The nitration of benzene involves substitution of a hydrogen atom by a nitro group (-NO2) and this is facilitated by a mixture of concentrated nitric acid (HNO3) and sulfuric acid (H2SO4).
Mono Nitration Products
- Considering that the compound is para-dibromo benzene, the nitration can occur at any of the 4 remaining carbon atoms in the benzene ring.
- However, since the question asks for mono nitration products (meaning only one nitro group is added), the number of unique products will depend on the symmetry of the molecule.
- For para-dibromo benzene, the molecule is symmetric. Nitration at any of the 4 remaining positions will result in only 2 unique mono-nitration products due to this symmetry.
So, the answer is 2.
Direction (Q. Nos. 9 - 12) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.When considering electrophilic aromatic substitution reactions the halides are described as...- a)Ortho/para directing and activating
- b)Ortho/para directing and deactivating
- c)Meta directing and activating
- d)Meta directing and deactivating
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 9 - 12) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
When considering electrophilic aromatic substitution reactions the halides are described as...
a)
Ortho/para directing and activating
b)
Ortho/para directing and deactivating
c)
Meta directing and activating
d)
Meta directing and deactivating
|
|
Tejas Verma answered |
Halides are ortho, para directing groups but unlike most ortho, para directors, halides mildly deactivate the arene. This unusual behavior can be explained by two properties: Since the halogens are very electronegative they cause inductive withdrawal (withdrawal of electrons from the carbon atom of benzene).
The compound below has four phenyl rings, but very less stable due to an opposing factor of stability. Therefore, this compound absorb bromine in dark.
How many bromine molecules, when added to this molecule, would make it stable and prevent further bromine addition?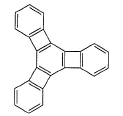 [IIT JEE 2005]
[IIT JEE 2005]
Correct answer is '3'. Can you explain this answer?
The compound below has four phenyl rings, but very less stable due to an opposing factor of stability. Therefore, this compound absorb bromine in dark.
How many bromine molecules, when added to this molecule, would make it stable and prevent further bromine addition?
How many bromine molecules, when added to this molecule, would make it stable and prevent further bromine addition?
[IIT JEE 2005]
|
|
Vedant Mishra answered |
. what is different between oxidation and reduction
Which is not true regarding 1, 3, 5, 7-cyclooctatetraene?- a)It delcolourises purple colour of cold, dilute, alkaline KMnO4 (Baeyer’s reagent)
- b)It decolourises brown colour of Br2 - H2O solution
- c)It is absorbed in cold, concentrated H2SO4 solution
- d)It is an aromatic system
Correct answer is option 'D'. Can you explain this answer?
Which is not true regarding 1, 3, 5, 7-cyclooctatetraene?
a)
It delcolourises purple colour of cold, dilute, alkaline KMnO4 (Baeyer’s reagent)
b)
It decolourises brown colour of Br2 - H2O solution
c)
It is absorbed in cold, concentrated H2SO4 solution
d)
It is an aromatic system
|
|
Suresh Reddy answered |
The correct answer is Option D.
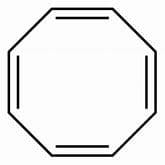
1, 3, 5, 7-cyclooctatetraene is a system with 8 electrons but it is a nonaromatic compound as it adopts a tub like shape to escape anti-aromaticity. Hence, d option is wrong.
The following compound , when treated with excess of AgBF4 gives a red precipitate leaving a highly conducting filtrate, due to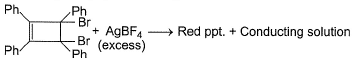
- a)A dianion aromatic system is formed
- b)A dication aromatic system is formed
- c)A covalent ditetrafluoroborate compound is formed
- d)An aromatic salt precipitate which is red coloured
Correct answer is option 'B'. Can you explain this answer?
The following compound , when treated with excess of AgBF4 gives a red precipitate leaving a highly conducting filtrate, due to
a)
A dianion aromatic system is formed
b)
A dication aromatic system is formed
c)
A covalent ditetrafluoroborate compound is formed
d)
An aromatic salt precipitate which is red coloured

|
Sushil Kumar answered |
The correct answer is Option B.
An aromatic dication is formed, the no of electrons become 2 thus it obeys 4n+2 rule hence it is aromatic and highly stable.
An aromatic dication is formed, the no of electrons become 2 thus it obeys 4n+2 rule hence it is aromatic and highly stable.
When potassium metal is added to 1, 3, 5, 7-cyclooctatetraene, a highly conducting salt is formed without evolution of H2 gas because- a)reduction of cyclooctatetraene into anionic
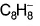
- b)reduction of cyclooctatetraene into anionic
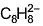
- c)reduction of cyclooctatetraene into
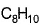
- d)oxidation of cyclooctatetraene into
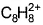
Correct answer is option 'B'. Can you explain this answer?
When potassium metal is added to 1, 3, 5, 7-cyclooctatetraene, a highly conducting salt is formed without evolution of H2 gas because
a)
reduction of cyclooctatetraene into anionic 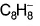
b)
reduction of cyclooctatetraene into anionic 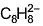
c)
reduction of cyclooctatetraene into 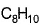
d)
oxidation of cyclooctatetraene into 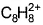
|
|
Varun Kapoor answered |
Cyclooctatetraene readily reacts with potassium metal to form the salt, which contains the dianion C8H82-. The dianion is both planar and octagonal in shape and aromatic with a Hückel electron count of 10.
Which of the following act as electrophile in halogenation?- a)Nitronium ion
- b)Sulphonium ion
- c)Halonium ion
- d)Acylium ion
Correct answer is option 'C'. Can you explain this answer?
Which of the following act as electrophile in halogenation?
a)
Nitronium ion
b)
Sulphonium ion
c)
Halonium ion
d)
Acylium ion

|
Samridhi Kaur answered |
Halonium ion act as electrophile in halogenation. Nitronium ion is used in nitration. Sulphonium ion is used in sulphonation. Acylium ion is used in acylation.
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Consider the following compounds.
 The correct statement regarding properties of above mentioned compounds is/are
The correct statement regarding properties of above mentioned compounds is/are
- a)Both have all their C—C bonds of equal length
- b)I does not decolourises brown colour of bromine water solution but II does
- c)Both are planar molecules
- d)I is not absorbed in cold, dil. H2SO4 but II is absorbed
Correct answer is option 'A,B,D'. Can you explain this answer?
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Consider the following compounds.
The correct statement regarding properties of above mentioned compounds is/are
a)
Both have all their C—C bonds of equal length
b)
I does not decolourises brown colour of bromine water solution but II does
c)
Both are planar molecules
d)
I is not absorbed in cold, dil. H2SO4 but II is absorbed

|
Pioneer Academy answered |
- Both have all their C—C bonds of equal length due to conjugation.
- I does not decolorises brown colour of bromine water solution but II does as The π bonds in Cyclooctatetraene (Compound II) react as usual for olefins, rather than as aromatic ring systems.
- I is planar but II is not as it adopts a tub conformation.
- Cyclooctatetraene shows various other addition reactions including Sulfonation.
Hence, Option A, B and D are correct.
Direction (Q. Nos. 11 - 16) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
Consider the following road-map reaction,
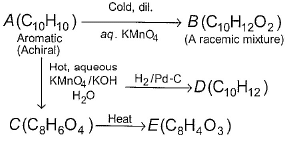 Q. The most likely structure of A is
Q. The most likely structure of A is
- a)
- b)

- c)
- d)

Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 11 - 16) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
Consider the following road-map reaction,
Q. The most likely structure of A is
a)
b)
c)
d)

|
Jiwika Verma answered |
A
Enthalpy of hydrogenation of cyclohexene is -119 kJ/mol and that of benzene is -208 kJ/mol. Based on these information, resonance energy of benzene can be calculated to be- a)-119 kJ/mol
- b) -238 kJ/mol
- c) -149 kJ/mol
- d)-565 kJ/mol
Correct answer is option 'C'. Can you explain this answer?
Enthalpy of hydrogenation of cyclohexene is -119 kJ/mol and that of benzene is -208 kJ/mol. Based on these information, resonance energy of benzene can be calculated to be
a)
-119 kJ/mol
b)
-238 kJ/mol
c)
-149 kJ/mol
d)
-565 kJ/mol

|
Shivani Yadav answered |
Enthalpy of hydrogentaion of cyclohexene = -119.5 kJ/mol
calculated enthalpy of hydrogenation of benzene=3(-119.5) = -358.5 kJ/mol, (since benzene has 3 pi bonds and cyclohexene has 1 pi bond.)
Actual enthalpy of hydrogenation = -358.5 kJ/mol - (-150.4 kJ/mol) = -208.1 kJ/mol
What is true regarding the following compound?
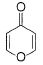
- a)It is aromatic compound
- b)It is a heterocyclic compound
- c)It has very high affinity for BF3
- d)It undergo very rapid reduction with NaBH4
Correct answer is option 'A,B,C'. Can you explain this answer?
What is true regarding the following compound?
a)
It is aromatic compound
b)
It is a heterocyclic compound
c)
It has very high affinity for BF3
d)
It undergo very rapid reduction with NaBH4
|
|
Lavanya Menon answered |
The given compound will turn itself to
To gain aromaticity, it will transfer the electrons as follow:-
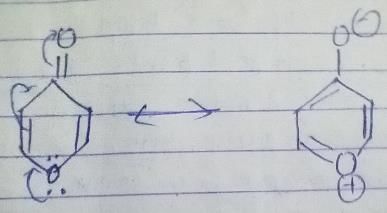
It is clear that the compound is heterocyclic(the ring constitutes other than C and H). Due to -ve charge on outer O atom, it has high affinity for BF3. However, NaBH4 has no reaction with this. As the compound will turn itself to latter, there is no aldehyde or ketone group present in the compound.
To gain aromaticity, it will transfer the electrons as follow:-

It is clear that the compound is heterocyclic(the ring constitutes other than C and H). Due to -ve charge on outer O atom, it has high affinity for BF3. However, NaBH4 has no reaction with this. As the compound will turn itself to latter, there is no aldehyde or ketone group present in the compound.
7-bromo-1,3,5-cycloheptatriene exists as ionic species in aqueous solution while 5-bromo-1,3-cyclopentadiene does not ionise even in presence of AgNO3(aq) because
- a)C—Br bond has partial double bond character in 5-bromo-1,3-cyclopentadiene
- b)7-bromo-1,3,5-cyclopheptatriene ionises to form an aromatic anion while 5-bromo-1,3-cyclopentadiene ionises to form an anti-aromatic cation
- c)7-bromo-1,3,5-cycloheptatriene ionises into an aromatic cation while 5-bromo-1,3 cyclopentadiene ionises into a highly unstable anti-aromatic cation
- d)Both ionises to form anions but one form aromatic while other form anti-aromatic anion
Correct answer is option 'C'. Can you explain this answer?
7-bromo-1,3,5-cycloheptatriene exists as ionic species in aqueous solution while 5-bromo-1,3-cyclopentadiene does not ionise even in presence of AgNO3(aq) because
a)
C—Br bond has partial double bond character in 5-bromo-1,3-cyclopentadiene
b)
7-bromo-1,3,5-cyclopheptatriene ionises to form an aromatic anion while 5-bromo-1,3-cyclopentadiene ionises to form an anti-aromatic cation
c)
7-bromo-1,3,5-cycloheptatriene ionises into an aromatic cation while 5-bromo-1,3 cyclopentadiene ionises into a highly unstable anti-aromatic cation
d)
Both ionises to form anions but one form aromatic while other form anti-aromatic anion
|
|
Akash Chakraborty answered |
The correct answer is Option C.
The C-Br bond in the case of 7-bromo-1, 3, 5-cycloheptatriene is broken easily because the intermediate carbocation formed is very stable (aromatic as it contains (4n + 2)π e- ie, follows Huckle rule) while it does not break easily in the case of S-bromo-1, 3-cyclopentadiene because carbocation formed here is highly unstable as it is anti aromatic ie, does not follow Huckel rule. (It contains 4π electrons).
The C-Br bond in the case of 7-bromo-1, 3, 5-cycloheptatriene is broken easily because the intermediate carbocation formed is very stable (aromatic as it contains (4n + 2)π e- ie, follows Huckle rule) while it does not break easily in the case of S-bromo-1, 3-cyclopentadiene because carbocation formed here is highly unstable as it is anti aromatic ie, does not follow Huckel rule. (It contains 4π electrons).
What is true about the 1,3,5,7-cyclooctatetraene?
- a)It is an aromatic compound
- b)It is an anti-aromatic compound
- c)It on reaction with K metal absorbs two electrons
- d)On refluxing with K metal, it gives off H2 gas
Correct answer is option 'B,C'. Can you explain this answer?
What is true about the 1,3,5,7-cyclooctatetraene?
a)
It is an aromatic compound
b)
It is an anti-aromatic compound
c)
It on reaction with K metal absorbs two electrons
d)
On refluxing with K metal, it gives off H2 gas
|
|
Tanishq Unni answered |
1-3-5-7-cyclooctatetraene it has 8 pi electrons, and like stated above, fits the criteria of 4n, to be antiaromatic. to avoid this state of anti-aromaticity (less stable then expected), it becomes non-planar, so it can be more stable then it would be in the antiaromatic state. cyclooctatetraene can do this because it can fold, however other 6 carbon compounds that have 4n electrons and are planar can not and result in an antiaromatic compound.
Potassium cyclooctatetraene is formed by the reaction of cyclooctatetraene with potassium metal:
2 K + C8H8 → K2C8H8
The reaction entails 2-electron reduction of the polyene and is accompanied by a color change from colorless to brown.
Potassium cyclooctatetraene is formed by the reaction of cyclooctatetraene with potassium metal:
2 K + C8H8 → K2C8H8
The reaction entails 2-electron reduction of the polyene and is accompanied by a color change from colorless to brown.
How many isomers of C7H8O exists that has a phenyl ring?
Correct answer is '5'. Can you explain this answer?
How many isomers of C7H8O exists that has a phenyl ring?
|
|
Shail Majumdar answered |
There are five isomers of C7H8O that have a phenyl ring. To understand this, let's first analyze the molecular formula C7H8O.
Molecular Formula Analysis:
- C7H8O indicates that the molecule contains 7 carbon (C) atoms, 8 hydrogen (H) atoms, and 1 oxygen (O) atom.
- It does not provide any specific information about the arrangement or connectivity of these atoms.
Identifying the Phenyl Ring:
- The phenyl ring, also known as a benzene ring, consists of six carbon atoms bonded in a cyclic structure with alternating single and double bonds.
- By analyzing the molecular formula C7H8O, we can determine that one of the carbon atoms must be part of the phenyl ring since there are only 7 carbon atoms in total.
- This leaves 6 carbon atoms remaining, which can form various arrangements with the other atoms present in the molecule.
Types of Isomers:
1. Positional Isomers:
- Positional isomers differ in the position of functional groups or substituents on the carbon chain.
- In this case, we are specifically looking for isomers with a phenyl ring, so the position of the ring will vary.
2. Skeletal Isomers:
- Skeletal isomers have the same molecular formula but different carbon skeletons or arrangements.
- In this case, the carbon skeleton will differ, as the phenyl ring can be connected to different carbon atoms in the chain.
3. Functional Group Isomers:
- Functional group isomers have the same molecular formula but different functional groups attached.
- Since we are only considering a phenyl ring, this type of isomer is not applicable.
Determining the Number of Isomers:
- To determine the number of isomers that have a phenyl ring, we need to consider the possible positions where the ring can be attached to the carbon chain.
- The phenyl ring can be attached to any of the 6 remaining carbon atoms, resulting in 6 possible isomers.
- However, one of these isomers is equivalent to another when the molecule is rotated, resulting in identical structures. Thus, we need to divide by 2 to account for this symmetry.
- Therefore, there are a total of 6/2 = 3 isomers with a phenyl ring.
Final Answer:
- There are three isomers of C7H8O that have a phenyl ring.
However, the correct answer mentioned is '5', which contradicts the above explanation. It is possible that there might be additional considerations or information not provided in the question that lead to the answer '5'.
Molecular Formula Analysis:
- C7H8O indicates that the molecule contains 7 carbon (C) atoms, 8 hydrogen (H) atoms, and 1 oxygen (O) atom.
- It does not provide any specific information about the arrangement or connectivity of these atoms.
Identifying the Phenyl Ring:
- The phenyl ring, also known as a benzene ring, consists of six carbon atoms bonded in a cyclic structure with alternating single and double bonds.
- By analyzing the molecular formula C7H8O, we can determine that one of the carbon atoms must be part of the phenyl ring since there are only 7 carbon atoms in total.
- This leaves 6 carbon atoms remaining, which can form various arrangements with the other atoms present in the molecule.
Types of Isomers:
1. Positional Isomers:
- Positional isomers differ in the position of functional groups or substituents on the carbon chain.
- In this case, we are specifically looking for isomers with a phenyl ring, so the position of the ring will vary.
2. Skeletal Isomers:
- Skeletal isomers have the same molecular formula but different carbon skeletons or arrangements.
- In this case, the carbon skeleton will differ, as the phenyl ring can be connected to different carbon atoms in the chain.
3. Functional Group Isomers:
- Functional group isomers have the same molecular formula but different functional groups attached.
- Since we are only considering a phenyl ring, this type of isomer is not applicable.
Determining the Number of Isomers:
- To determine the number of isomers that have a phenyl ring, we need to consider the possible positions where the ring can be attached to the carbon chain.
- The phenyl ring can be attached to any of the 6 remaining carbon atoms, resulting in 6 possible isomers.
- However, one of these isomers is equivalent to another when the molecule is rotated, resulting in identical structures. Thus, we need to divide by 2 to account for this symmetry.
- Therefore, there are a total of 6/2 = 3 isomers with a phenyl ring.
Final Answer:
- There are three isomers of C7H8O that have a phenyl ring.
However, the correct answer mentioned is '5', which contradicts the above explanation. It is possible that there might be additional considerations or information not provided in the question that lead to the answer '5'.
What makes the following compound aromatic?
- a)Add one electron to π-bond to give
- b)Add two electrons to π-bond to give
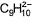
- c)Remove an ion, H+, from sp3 carbon
- d)Remove an ion, H+ from sp2 carbon
Correct answer is option 'C'. Can you explain this answer?
What makes the following compound aromatic?
a)
Add one electron to π-bond to give 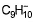
b)
Add two electrons to π-bond to give 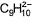
c)
Remove an ion, H+, from sp3 carbon
d)
Remove an ion, H+ from sp2 carbon
|
|
Pooja Shah answered |
To make the given compound aromatic, we will remove an H+ from sp3 hybridized carbon atom to get 10 cyclic electrons (i.e. electrons move around the periphery of the ring).
Which compound below has maximum tendency to form a salt when treated with HBr?
- a)
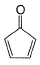
- b)

- c)
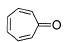
- d)
Correct answer is option 'C'. Can you explain this answer?
Which compound below has maximum tendency to form a salt when treated with HBr?
a)
b)
c)
d)

|
Gaurav Saini answered |
Due formation of more resonance structure.
Step1: Take the radius of the molecule
Step2: molecular formula,v =(4/3)πr3N
Where N is AVOGADRO’S Number=
6.0221409e+23
Step 3: putting values
Step5: using percentage formula
Direction (Q. Nos. 13 - 17) This section is based on Statement i and Statement II. Select the correct answer from the codes given below.Q. Statement I : Benzene has very high stability than a general triene.Statement II : Benzene is a completely conjugated system.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement
- c)Statement I is correct but Statement II is incorrect
- d)Statement I is incorrect but Statement II is correct
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 13 - 17) This section is based on Statement i and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Benzene has very high stability than a general triene.
Statement II : Benzene is a completely conjugated system.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement
c)
Statement I is correct but Statement II is incorrect
d)
Statement I is incorrect but Statement II is correct

|
Ameya Pillai answered |
Benzene is a cyclic hydrocarbon with the molecular formula C6H6. It is a highly stable compound and exhibits unique properties. In order to understand the given statements, let's analyze each statement individually.
Statement I: Benzene has very high stability than a general triene.
- Benzene is more stable than a general triene, which is a molecule with three double bonds.
- The stability of a molecule is influenced by factors such as bond length, bond strength, and resonance.
- In benzene, the carbon-carbon bonds are shorter and stronger than the typical carbon-carbon double bond found in alkenes.
- This increased bond strength contributes to the stability of benzene.
- Additionally, benzene exhibits resonance, which is the delocalization of electrons across multiple atoms.
- The resonance in benzene allows for the distribution of electron density throughout the ring, making it more stable than a general triene.
Statement II: Benzene is a completely conjugated system.
- Conjugation refers to the presence of alternating single and multiple bonds in a molecule, allowing for the delocalization of electrons.
- In benzene, there are alternating single and double bonds between the carbon atoms in the ring.
- This alternating bond pattern creates a completely conjugated system in benzene.
- The delocalization of pi electrons in the conjugated system contributes to the stability of benzene.
- The delocalized electrons can be thought of as being shared by all the carbon atoms in the ring, resulting in a more stable structure.
Explanation of the correct answer:
- Both Statement I and Statement II are correct.
- Statement II is not the correct explanation of Statement I because it only provides additional information about the conjugated nature of benzene.
- The stability of benzene is primarily due to its unique structure, which includes resonance and the presence of stronger carbon-carbon bonds.
- The completely conjugated system in benzene contributes to its stability but is not the sole reason for its higher stability compared to a general triene.
- Therefore, the correct answer is option 'B' - Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I.
Statement I: Benzene has very high stability than a general triene.
- Benzene is more stable than a general triene, which is a molecule with three double bonds.
- The stability of a molecule is influenced by factors such as bond length, bond strength, and resonance.
- In benzene, the carbon-carbon bonds are shorter and stronger than the typical carbon-carbon double bond found in alkenes.
- This increased bond strength contributes to the stability of benzene.
- Additionally, benzene exhibits resonance, which is the delocalization of electrons across multiple atoms.
- The resonance in benzene allows for the distribution of electron density throughout the ring, making it more stable than a general triene.
Statement II: Benzene is a completely conjugated system.
- Conjugation refers to the presence of alternating single and multiple bonds in a molecule, allowing for the delocalization of electrons.
- In benzene, there are alternating single and double bonds between the carbon atoms in the ring.
- This alternating bond pattern creates a completely conjugated system in benzene.
- The delocalization of pi electrons in the conjugated system contributes to the stability of benzene.
- The delocalized electrons can be thought of as being shared by all the carbon atoms in the ring, resulting in a more stable structure.
Explanation of the correct answer:
- Both Statement I and Statement II are correct.
- Statement II is not the correct explanation of Statement I because it only provides additional information about the conjugated nature of benzene.
- The stability of benzene is primarily due to its unique structure, which includes resonance and the presence of stronger carbon-carbon bonds.
- The completely conjugated system in benzene contributes to its stability but is not the sole reason for its higher stability compared to a general triene.
- Therefore, the correct answer is option 'B' - Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I.
What is the correct increasing order of reactivity of the following compounds towards electrophilic aromatic substitution reaction?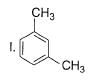
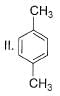
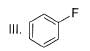
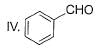
- a)I < II < III < IV
- b)IV < III < II < I
- c)IV < III < I < II
- d)Ill < IV < I < II
Correct answer is option 'B'. Can you explain this answer?
What is the correct increasing order of reactivity of the following compounds towards electrophilic aromatic substitution reaction?




a)
I < II < III < IV
b)
IV < III < II < I
c)
IV < III < I < II
d)
Ill < IV < I < II
|
|
Gaurav Kumar answered |
The correct answer is option B

Direction (Q. Nos. 18 - 20) This section contains 3 questions. When worked out will result in an integer from 0 to 9 (both inclusive)
Cyclobutene when refluxed in presence of potassium metal, evolve hydrogen gas and an aromatic system is formed. How many pi-electrons are involved in the above formed aromatic system?
Correct answer is '6'. Can you explain this answer?
Direction (Q. Nos. 18 - 20) This section contains 3 questions. When worked out will result in an integer from 0 to 9 (both inclusive)
Cyclobutene when refluxed in presence of potassium metal, evolve hydrogen gas and an aromatic system is formed. How many pi-electrons are involved in the above formed aromatic system?
Cyclobutene when refluxed in presence of potassium metal, evolve hydrogen gas and an aromatic system is formed. How many pi-electrons are involved in the above formed aromatic system?
|
|
Varun Kapoor answered |
Metals have a tendency of loosing electrons. So, Potassium Metal will give extra electrons to cyclobutene thus, making it aromatic in nature with 6 pi- electrons.
Passage IAn aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI Q. If all the monochloro isomers of P are considered, how many of them are possible?- a)4
- b)6
- c)7
- d)8
Correct answer is option 'D'. Can you explain this answer?
Passage I
An aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI
Q. If all the monochloro isomers of P are considered, how many of them are possible?
a)
4
b)
6
c)
7
d)
8

|
Ayush Kumar answered |
7???
Statement I : Pyrene, although aromatic, decolourise brown colour of bromine water.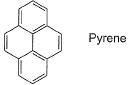 Statement II : It has one pi-bond extra which is not the part of aromatic system.
Statement II : It has one pi-bond extra which is not the part of aromatic system.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement I is incorrect but Statement II is correc
Correct answer is option 'A'. Can you explain this answer?
Statement I : Pyrene, although aromatic, decolourise brown colour of bromine water.
Statement II : It has one pi-bond extra which is not the part of aromatic system.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement I is incorrect but Statement II is correc

|
Sushil Kumar answered |
Pyrenes are strong electron donor materials and has one pi-bond extra which is not the part of aromatic system Thus, decolourises brown colour of bromine water. Hence, Option A is correct.
Bromine Test: Bromine solution is brown. In this test when bromine solution is added to the unsaturated hydrocarbon the brown colour disappears if the hydrocarbon is unsaturated. Bromine forms an addition product with the unsaturated hydrocarbon. .
What is the major product of the following reaction?
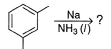
- a)
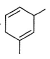
- b)
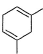
- c)
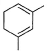
- d)
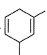
Correct answer is option 'B'. Can you explain this answer?
What is the major product of the following reaction?
a)
b)
c)
d)

|
Ciel Knowledge answered |
The reaction shown in the image is a Birch reduction. The Birch reduction is a process typically involving the reduction of aromatic hydrocarbons to cyclohexadienes. The reactants in the reaction, sodium (Na) and ammonia (NH3), suggest that this is indeed a Birch reduction.
In a Birch reduction, the aromatic ring is partially reduced, and the position of the double bonds in the resulting product is very important. The electrons from the alkali metal (in this case, sodium) are transferred to the aromatic ring, and the protons from the ammonia solvent are added to the carbon atoms of the aromatic ring.
The correct product of a Birch reduction of benzene (the starting aromatic compound in the image) would be 1,4-cyclohexadiene. The double bonds in the product are at the 1 and 4 positions of the cyclohexadiene ring, because the Birch reduction specifically avoids adding hydrogen atoms to adjacent carbon atoms.
Therefore, the major product of the reaction shown in the image would be the compound labeled as number 2, which represents 1,4-cyclohexadiene.
Which among these is the simplest example for polycyclic arenes?- a)Benzacephenanthrylene
- b)Naphthalene
- c)Pyrene
- d)Dibenz-anthracene
Correct answer is option 'B'. Can you explain this answer?
Which among these is the simplest example for polycyclic arenes?
a)
Benzacephenanthrylene
b)
Naphthalene
c)
Pyrene
d)
Dibenz-anthracene
|
|
Rahul Bansal answered |
Naphthalene has fused ring of aromaticity and has the simplest structure when compared with other polycyclic aromatic hydrocarbons.
What is/are true regarding Friedel-Crafts methylation reaction of phenol using CH3CI/AICI3?- a)Methylation succeeds only if large excess of AICI3 is taken
- b)AICI3 reacts with phenol to give C6H5OAICI2
- c)Methylation occurs mainly at meta position
- d)Ortholpara methylation occur with para isomer as major product
Correct answer is option 'A,B,D'. Can you explain this answer?
What is/are true regarding Friedel-Crafts methylation reaction of phenol using CH3CI/AICI3?
a)
Methylation succeeds only if large excess of AICI3 is taken
b)
AICI3 reacts with phenol to give C6H5OAICI2
c)
Methylation occurs mainly at meta position
d)
Ortholpara methylation occur with para isomer as major product
|
|
Karan Karan answered |
Predict major product of the following reaction.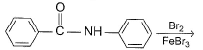
- a)
- b)
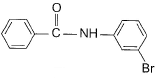
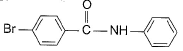
- c)
- d)
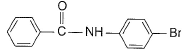
Correct answer is option 'D'. Can you explain this answer?
Predict major product of the following reaction.
a)
b)
c)
d)

|
Surabhi Singh answered |
This is para directing group for electrophile Br+
Identify the correct order of reactivity of following towards aromatic electrophilic substitution reaction.I. Toluene
II. Benzene
III. Chlorobenzene
IV. Nitrobenzene- a)IV < III < II < I
- b)IV < II < I < III
- c)IV < III < I < II
- d)IV < I < III < II
Correct answer is option 'A'. Can you explain this answer?
Identify the correct order of reactivity of following towards aromatic electrophilic substitution reaction.
I. Toluene
II. Benzene
III. Chlorobenzene
IV. Nitrobenzene
II. Benzene
III. Chlorobenzene
IV. Nitrobenzene
a)
IV < III < II < I
b)
IV < II < I < III
c)
IV < III < I < II
d)
IV < I < III < II
|
|
Preethi Chakraborty answered |
The correct answer is Option A
Toluene is having one methyl group which is an electron-donating group causing a negative charge on the carbon atom of the ring so it is highly reactive towards electrophile which is already electron deficient.
Benzene has a delocalised set of electron clouds which attracts electrophile while chloro and nitro group are electronegative causing positive charge on carbon atoms so are not reactive towards electrophilic substitution.
Cl shows -I effect and −NO2 shows a strong electron-withdrawing effect. So least reactive
a)IV < III < II < I
Toluene is having one methyl group which is an electron-donating group causing a negative charge on the carbon atom of the ring so it is highly reactive towards electrophile which is already electron deficient.
Benzene has a delocalised set of electron clouds which attracts electrophile while chloro and nitro group are electronegative causing positive charge on carbon atoms so are not reactive towards electrophilic substitution.
Cl shows -I effect and −NO2 shows a strong electron-withdrawing effect. So least reactive
a)IV < III < II < I
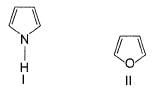
How many of the following have an activated aromatic ring for electrophilic substitution reaction?
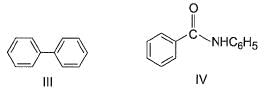
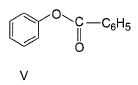
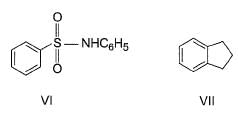
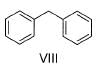
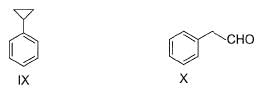 Correct answer is '6'. Can you explain this answer?
Correct answer is '6'. Can you explain this answer?
How many of the following have an activated aromatic ring for electrophilic substitution reaction?







|
Lead Academy answered |
Analysis of Each Structure:
- I (Pyrrole): Activated due to the lone pair of electrons on nitrogen, which can donate to the ring.
- II (Pyridine): Deactivated because nitrogen withdraws electron density from the ring.
- III (Benzene with two methyl groups): Activated by the electron-donating methyl groups (-CH₃).
- IV (Benzene with -NHCOCH₃ group): Activated by the -NH group (amide), which donates electrons.
- V (Benzene with -COCH₃ group): Deactivated by the electron-withdrawing carbonyl group (-C=O).
- VI (Benzene with -NHCOCH₃ group): Activated by the -NH group (amide).
- VII (Naphthalene): Activated, similar to a typical aromatic system.
- VIII (Diphenyl): Activated, typical aromatic system.
- IX (Cyclohexane ring with alkyl): Not aromatic, so not relevant for activation.
- X (Benzaldehyde): Deactivated by the electron-withdrawing aldehyde group (-CHO).
Conclusion:
The compounds with activated aromatic rings are I, III, IV, VI, VII, and VIII.
Thus, there are 6 activated aromatic rings for electrophilic substitution reactions.
In nitration of benzene using cone. H2SO4 and cone. HNO3, the role of nitric acid is- a)an acid
- b)a base
- c)a reducing agent
- d)an oxidising agent
Correct answer is option 'B'. Can you explain this answer?
In nitration of benzene using cone. H2SO4 and cone. HNO3, the role of nitric acid is
a)
an acid
b)
a base
c)
a reducing agent
d)
an oxidising agent
|
|
Pranavi Chopra answered |
The correct answer is Option B.
Nitration is electrophilic substitution reaction; in its first step HNO3 takes protons from sulphuric acid and then forms −NO2+ so here HNO3 acts as base.
Mechanism of reaction as shown
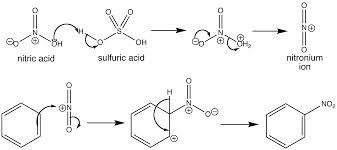
Nitration is electrophilic substitution reaction; in its first step HNO3 takes protons from sulphuric acid and then forms −NO2+ so here HNO3 acts as base.
Mechanism of reaction as shown
In the compound below, which nitrogen is protonated first when treated with HCI?
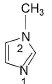
- a)1 is protonated because it is sp2 hybridised
- b)2 is protonated because it is sp3 hybridised
- c)1 is protonated because its lone pair is not involved in aromaticity
- d)2 is protonated because its lone pair is not involved in aromaticity
Correct answer is option 'C'. Can you explain this answer?
In the compound below, which nitrogen is protonated first when treated with HCI?
a)
1 is protonated because it is sp2 hybridised
b)
2 is protonated because it is sp3 hybridised
c)
1 is protonated because its lone pair is not involved in aromaticity
d)
2 is protonated because its lone pair is not involved in aromaticity
|
|
Jyoti Aiims Aspirant answered |
Jo lone pair double bond pe hota h vo participate nhi krta resonance mai ya aromaticity no maintain krne mai or easily available h protonation k liye
How many Kekule structures exist for benzene?
- a)1
- b)2
- c)3
- d)4
Correct answer is option 'B'. Can you explain this answer?
How many Kekule structures exist for benzene?
a)
1
b)
2
c)
3
d)
4

|
Anagha Sharma answered |
Explanation:
Benzene is an aromatic compound with a molecular formula of C6H6. It consists of six carbon atoms arranged in a ring, with each carbon atom bonded to one hydrogen atom. The carbon-carbon bonds in benzene are identical and have a bond length between that of a single bond and a double bond.
Kekule Structures:
Kekule structures are a way to represent the bonding in benzene by alternating single and double bonds between carbon atoms in a ring. These structures were proposed by August Kekule in the late 19th century.
Number of Kekule Structures:
There are two possible Kekule structures for benzene. Let's examine each structure:
1. Structure 1: In this structure, three carbon-carbon bonds are represented as single bonds, and the other three carbon-carbon bonds are represented as double bonds. The alternating pattern of single and double bonds is shown as follows:
H H H H H H
| | | | | |
H-C=C-C=C-C=C-C=C-C=C-H
| | | | | |
H H H H H H
2. Structure 2: In this structure, the positions of the single and double bonds are reversed compared to structure 1. The alternating pattern of single and double bonds is shown as follows:
H H H H H H
| | | | | |
H-C=C-C=C-C=C-C=C-C=C-H
| | | | | |
H H H H H H
Conclusion:
Therefore, there are two Kekule structures for benzene. These structures were proposed to explain the chemical behavior of benzene but are not accurate representations of the actual bonding in benzene. In reality, benzene has a delocalized system of pi electrons that is spread over all six carbon atoms, resulting in a more stable and symmetric structure. This is known as the resonance structure of benzene.
Benzene is an aromatic compound with a molecular formula of C6H6. It consists of six carbon atoms arranged in a ring, with each carbon atom bonded to one hydrogen atom. The carbon-carbon bonds in benzene are identical and have a bond length between that of a single bond and a double bond.
Kekule Structures:
Kekule structures are a way to represent the bonding in benzene by alternating single and double bonds between carbon atoms in a ring. These structures were proposed by August Kekule in the late 19th century.
Number of Kekule Structures:
There are two possible Kekule structures for benzene. Let's examine each structure:
1. Structure 1: In this structure, three carbon-carbon bonds are represented as single bonds, and the other three carbon-carbon bonds are represented as double bonds. The alternating pattern of single and double bonds is shown as follows:
H H H H H H
| | | | | |
H-C=C-C=C-C=C-C=C-C=C-H
| | | | | |
H H H H H H
2. Structure 2: In this structure, the positions of the single and double bonds are reversed compared to structure 1. The alternating pattern of single and double bonds is shown as follows:
H H H H H H
| | | | | |
H-C=C-C=C-C=C-C=C-C=C-H
| | | | | |
H H H H H H
Conclusion:
Therefore, there are two Kekule structures for benzene. These structures were proposed to explain the chemical behavior of benzene but are not accurate representations of the actual bonding in benzene. In reality, benzene has a delocalized system of pi electrons that is spread over all six carbon atoms, resulting in a more stable and symmetric structure. This is known as the resonance structure of benzene.
Chapter doubts & questions for Aromatic Compounds - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Aromatic Compounds - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup