All Exams >
MCAT >
General Chemistry for MCAT >
All Questions
All questions of Chemical Equilibrium for MCAT Exam
Which of the following statements most accurately describes the comparison between the rate constant k and the equilibrium constant keq?- a)Both constants are dependent on temperature, but only Keq is independent of concentration.
- b)The rate constant k indicates how long the reaction will take to complete, and the equilibrium constant Keq indicates the ratio of the product concentrations to the reactant concentrations at equilibrium.
- c)A large k means that the reaction will go to equilibrium, and a large Keq means that a reaction will go to completion quickly.
- d)k is formed from the concentration of the reactants raised to their stoichiometric coefficients, while Keq is formed from the concentration of the products over the reactants each raised to its stoichiometric coefficient.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements most accurately describes the comparison between the rate constant k and the equilibrium constant keq?
a)
Both constants are dependent on temperature, but only Keq is independent of concentration.
b)
The rate constant k indicates how long the reaction will take to complete, and the equilibrium constant Keq indicates the ratio of the product concentrations to the reactant concentrations at equilibrium.
c)
A large k means that the reaction will go to equilibrium, and a large Keq means that a reaction will go to completion quickly.
d)
k is formed from the concentration of the reactants raised to their stoichiometric coefficients, while Keq is formed from the concentration of the products over the reactants each raised to its stoichiometric coefficient.
|
|
Ayesha Joshi answered |
Let’s evaluate each statement individually. A large rate constant k means that the reaction will go to equilibrium quickly, while a large equilibrium constant Keq means that the forward reaction will go to completion, and the concentration of products will exceed that of the reactants.
Keq is formed from the concentrations of the reactants each raised to their stoichiometric coefficients, while k is formed from the concentrations of the reactants raised to the stoichiometric coefficients only if dealing with elementary reactions. Otherwise, the order of the reaction must be determined by experimental data.
The rate constant indicates how quickly it will take to reach equilibrium, which is similar to how long the reaction will take to reach completion. The Keq does give an indication of the relative concentrations of the reactants versus the products at equilibrium. If we are talking about ratio, then its the concentrations set to their stoichiometric coefficients.
The correct answer is that both constants are dependent on temperature. Keq is independent of concentration. Once at equilibrium, if the concentrations of the reactants and products change, they will readjust such that the ratio equals Keq again. Rate is not independent of concentration; the greater the concentration, the greater the initial speed of reaction.
The Haber process involves the production of ammonia from hydrogen and nitrogen gas. In the laboratory, it was determined that the equilibrium concentrations of NH3, H2 and N2 are 0.0030 M, 0.10 M , and 0.090 M, respectively. Which of the following statements most accurately describes the reaction progress when all three concentrations are at 0.3 and 3.0 M?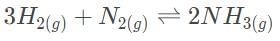
- a)For both concentrations, the reaction will shift to the right.
- b)For 3.0 M, the reaction will shift to the left, and for 0.3 M, the reaction will shift to the right.
- c)For both concentrations, the reaction will shift to the left.
- d)For 0.3 M, the reaction will shift to the right, and for 3.0 M, the reaction will shift to the left.
Correct answer is option 'C'. Can you explain this answer?
The Haber process involves the production of ammonia from hydrogen and nitrogen gas. In the laboratory, it was determined that the equilibrium concentrations of NH3, H2 and N2 are 0.0030 M, 0.10 M , and 0.090 M, respectively. Which of the following statements most accurately describes the reaction progress when all three concentrations are at 0.3 and 3.0 M?
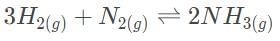
a)
For both concentrations, the reaction will shift to the right.
b)
For 3.0 M, the reaction will shift to the left, and for 0.3 M, the reaction will shift to the right.
c)
For both concentrations, the reaction will shift to the left.
d)
For 0.3 M, the reaction will shift to the right, and for 3.0 M, the reaction will shift to the left.

|
Orion Classes answered |
Since the equilibrium concentrations of the reactants and products are given, the value of Keq can be calculated:
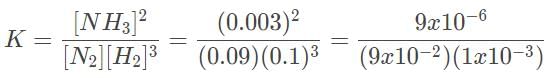 = 0.1
= 0.1Let’s evaluate when all the concentrations are 0.3 M using the reaction quotient Q, which has the same formula as Keq:
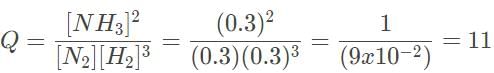
Let’s evaluate when all the concentrations are 3.0 M using the reaction quotient Q, which has the same formula as Keq:
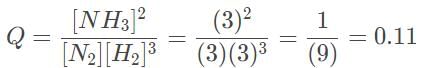
Since Q > Keq for both, the reaction will shift to the left.
What effect does increasing the temperature have on an exothermic reaction at equilibrium?- a)The equilibrium will shift towards the reactants.
- b)The equilibrium will shift towards the products.
- c)The equilibrium will remain unchanged.
- d)The equilibrium will be disrupted.
Correct answer is option 'A'. Can you explain this answer?
What effect does increasing the temperature have on an exothermic reaction at equilibrium?
a)
The equilibrium will shift towards the reactants.
b)
The equilibrium will shift towards the products.
c)
The equilibrium will remain unchanged.
d)
The equilibrium will be disrupted.
|
|
Ayesha Joshi answered |
An exothermic reaction releases heat, and increasing the temperature is a stress on the equilibrium. According to Le Chatelier's principle, the system will respond by shifting in a direction that counteracts the stress, which in this case is towards the reactants.
Which of the following is a true statement about the role of catalysts in a reaction?
I. Catalysts more effectively lowers the activation energy in the forward direction.
II. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product..
III. If a catalyst affects the equilibrium of the reaction, it must be consumed as the reaction proceeds.
IV. Catalysts can may increase the reaction rate or selectivity or enable the reaction at a lower temperature.- a)II, III, and IV
- b)II and IV
- c)I and III
- d)II and III
Correct answer is option 'B'. Can you explain this answer?
Which of the following is a true statement about the role of catalysts in a reaction?
I. Catalysts more effectively lowers the activation energy in the forward direction.
II. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product..
III. If a catalyst affects the equilibrium of the reaction, it must be consumed as the reaction proceeds.
IV. Catalysts can may increase the reaction rate or selectivity or enable the reaction at a lower temperature.
I. Catalysts more effectively lowers the activation energy in the forward direction.
II. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product..
III. If a catalyst affects the equilibrium of the reaction, it must be consumed as the reaction proceeds.
IV. Catalysts can may increase the reaction rate or selectivity or enable the reaction at a lower temperature.
a)
II, III, and IV
b)
II and IV
c)
I and III
d)
II and III
|
|
Ayesha Joshi answered |
Analyze each statement and eliminate any answer choices as we move along.
Catalysts more effectively lowers the activation energy in the forward reaction is an incorrect statement. If we look at any energy profile, it will become apparent that by lowering the activation energy of the forward reaction that of the reverse reaction is also lowered.
Catalysts generally react with one or more reactants to form intermediates that subsequently given the final reaction product is a true statement. What needs further qualification is the process of giving the final reaction product. In that process, what is regenerated is the catalyst. A catalyst may react with the reactants as long as it is regenerated by the reaction.
If a catalyst affects the equilibrium of the reaction, it must be consumed as the reaction proceeds is an incorrect statement. Catalysts can only affect the kinetics or the rate at which the reaction approaches equilibrium, but not the equilibrium constant of the reaction. Concentration, pressure, temperature can affect the equilibrium, but not a catalyst.
Catalysts may increase the reaction rate or selectivity or enable the reaction at a lower temperature. Lowering the activation energy would allow for the reaction to occur at a lower temperature since less heat is needed to overcome the barrier. Statement IV is correct.
Statements II and IV are correct.
What effect does increasing the pressure have on a system at equilibrium involving only gases?- a)The equilibrium will shift towards the side with fewer moles of gas.
- b)The equilibrium will shift towards the side with more moles of gas.
- c)The equilibrium will remain unchanged.
- d)The equilibrium will be disrupted.
Correct answer is option 'B'. Can you explain this answer?
What effect does increasing the pressure have on a system at equilibrium involving only gases?
a)
The equilibrium will shift towards the side with fewer moles of gas.
b)
The equilibrium will shift towards the side with more moles of gas.
c)
The equilibrium will remain unchanged.
d)
The equilibrium will be disrupted.
|
|
Ayesha Joshi answered |
Increasing the pressure on a system at equilibrium involving only gases is a stress on the equilibrium. According to Le Chatelier's principle, the system will respond by shifting in a direction that reduces the pressure. This means that the equilibrium will shift towards the side with more moles of gas, as this will result in a decrease in the total pressure.
What happens to the value of the equilibrium constant (K) if the coefficients of a balanced chemical equation are multiplied by a factor?- a)The value of K remains unchanged.
- b)The value of K increases.
- c)The value of K decreases.
- d)The value of K becomes zero.
Correct answer is option 'B'. Can you explain this answer?
What happens to the value of the equilibrium constant (K) if the coefficients of a balanced chemical equation are multiplied by a factor?
a)
The value of K remains unchanged.
b)
The value of K increases.
c)
The value of K decreases.
d)
The value of K becomes zero.
|
|
Ayesha Joshi answered |
If the coefficients of a balanced chemical equation are multiplied by a factor, the value of the equilibrium constant (K) is raised to the power of that factor. This means that if the coefficients are multiplied by n, the value of K will be raised to the power of n. Therefore, the value of K increases.
Which of the following statements is true regarding a system at equilibrium?- a)The rates of the forward and reverse reactions are equal.
- b)The concentrations of reactants and products are equal.
- c)The system has stopped changing.
- d)The reaction has come to a halt.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements is true regarding a system at equilibrium?
a)
The rates of the forward and reverse reactions are equal.
b)
The concentrations of reactants and products are equal.
c)
The system has stopped changing.
d)
The reaction has come to a halt.
|
|
Ayesha Joshi answered |
In a system at equilibrium, the rates of the forward and reverse reactions are equal. While the concentrations of reactants and products may not be equal, their relative concentrations remain constant over time.
How does decreasing the temperature affect an endothermic reaction at equilibrium?- a)The equilibrium will shift towards the reactants.
- b)The equilibrium will shift towards the products.
- c)The equilibrium will remain unchanged.
- d)The equilibrium will be disrupted.
Correct answer is option 'A'. Can you explain this answer?
How does decreasing the temperature affect an endothermic reaction at equilibrium?
a)
The equilibrium will shift towards the reactants.
b)
The equilibrium will shift towards the products.
c)
The equilibrium will remain unchanged.
d)
The equilibrium will be disrupted.
|
|
Ayesha Joshi answered |
An endothermic reaction absorbs heat, and decreasing the temperature is a stress on the equilibrium. According to Le Chatelier's principle, the system will respond by shifting in a direction that counteracts the stress, which in this case is towards the reactants.
Which of the following is considered necessary as part of standard state conditions?- a)pH 7 for all solutions
- b)ambient pressure of 100,000 Pascals
- c)1 Molar for all solutions, including water
- d)ambient temperature of 273 K or 310 K
Correct answer is option 'B'. Can you explain this answer?
Which of the following is considered necessary as part of standard state conditions?
a)
pH 7 for all solutions
b)
ambient pressure of 100,000 Pascals
c)
1 Molar for all solutions, including water
d)
ambient temperature of 273 K or 310 K
|
|
Ayesha Joshi answered |
Standard state conditions should not be confused with standard temperature and pressure for gases or standard conditions for procedures carried out in laboratory settings. Standard state conditions is commonly used in the thermodynamic evaluations of a reaction.
Most tables of thermodynamic quantities are collected at one of two temperatures, 273 K or 298 K more commonly. Standard state does not technically specify a temperature.
For a substance in solution, the standard state molality is 1 mol kg−1 while standard state amount concentration is 1 mol dm−3. It is assumed that water does not change its concentration of 55.6 M appreciably.
The pH of a solution is not mentioned in standard state conditions.
Some of us may be more familiar with seeing 1 atmosphere, but IUPAC does recommend a standard pressure of 105 Pascals for standard state conditions. Since 105 Pascals is 0.99 atmospheres, they are essentially the same. Therefore, an ambient pressure of 100,000 Pascals is necessary as part of the standard state conditions.
Consider the following reaction: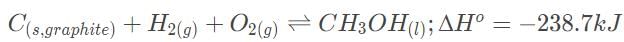 Which of the following would increase the value of Keq?
Which of the following would increase the value of Keq?- a)Increase [H2]
- b)Increase pressure
- c)Decrease [C]
- d)Decrease temperature
Correct answer is option 'D'. Can you explain this answer?
Consider the following reaction:
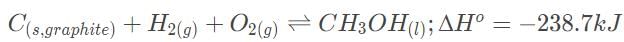
Which of the following would increase the value of Keq?
a)
Increase [H2]
b)
Increase pressure
c)
Decrease [C]
d)
Decrease temperature
|
|
Ayesha Joshi answered |
Increasing the Keq will increase the amount of product formed at equilibrium. When we increase the pressure, the equilibrium will shift to the right according to Le Chatelier’s Principle. The concentrations will change, but will readjust such that Keq remains the same.
Increasing or decreasing the concentration will cause the equilibrium to shift to the right or left, respectively, but the value of Keq remains constant since it is concentration-independent.
Only by increasing or decreasing the temperature will change the value of Keq since Keq = e(-ΔG/RT).
By decreasing the temperature for an exothermic reaction, the equilibrium will shift to the right such that the concentration of the products increase and the concentration of the reactants decrease. That results in an increased value of Keq
Chapter doubts & questions for Chemical Equilibrium - General Chemistry for MCAT 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Equilibrium - General Chemistry for MCAT in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
General Chemistry for MCAT
164 videos|11 docs|16 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









