All Exams >
MCAT >
MCAT Mock Test Series 2025 >
All Questions
All questions of Chemistry for MCAT Exam
The boiling point of benzene is 80°C. Estimate its molar heat of vaporization. Assume that it obeys Trouton's rule.- a)25.64 kJ mol-1
- b)31.064 kJ mol-1
- c)29.96 kJ mol-1
- d)39.54 kJ mol-1
- e)40.67 kJ mol-1
Correct answer is option 'B'. Can you explain this answer?
The boiling point of benzene is 80°C. Estimate its molar heat of vaporization. Assume that it obeys Trouton's rule.
a)
25.64 kJ mol-1
b)
31.064 kJ mol-1
c)
29.96 kJ mol-1
d)
39.54 kJ mol-1
e)
40.67 kJ mol-1
|
|
Ayesha Joshi answered |
From the Trouton's law ΔH/Tb = 88 J mol-1 K-1
The given data is Tb = 80°C = 80 + 273 = 353 K
Therefore, ΔH = (88J mol-1 K-1) (353 K) = 31064 J mol-1 = 31.064 kJ mol-1
The given data is Tb = 80°C = 80 + 273 = 353 K
Therefore, ΔH = (88J mol-1 K-1) (353 K) = 31064 J mol-1 = 31.064 kJ mol-1
Calculate the angle at which second order reflection will occur in an X-ray spectrometer when X-rays of wavelength 1.54λ are diffracted by atoms of a crystal, with interplanar distance of 4.04 A0.- a)100 59'
- b)220 24'
- c)240 22'
- d)590 10'
- e)120 50'
Correct answer is option 'B'. Can you explain this answer?
Calculate the angle at which second order reflection will occur in an X-ray spectrometer when X-rays of wavelength 1.54λ are diffracted by atoms of a crystal, with interplanar distance of 4.04 A0.
a)
100 59'
b)
220 24'
c)
240 22'
d)
590 10'
e)
120 50'
|
|
Emily Lewis answered |
Calculation of Second Order Reflection Angle in X-ray Spectrometer:
- Given:
- Wavelength of X-rays, λ = 1.54 Å
- Interplanar distance of crystal, d = 4.04 Å
- Order of reflection, n = 2
Formula:
The angle of reflection for X-rays in a crystal can be calculated using Bragg's Law:
nλ = 2d sinθ
Calculation:
- Substituting the given values into the formula:
2 * 1.54 Å = 2 * 4.04 Å * sinθ
3.08 Å = 8.08 Å * sinθ
sinθ = 3.08 Å / 8.08 Å
sinθ = 0.381
- Calculating the angle:
θ = sin⁻¹(0.381)
θ ≈ 22°
Therefore, the angle at which the second order reflection will occur in the X-ray spectrometer is approximately 22°. This corresponds to option 'b) 22°'.
- Given:
- Wavelength of X-rays, λ = 1.54 Å
- Interplanar distance of crystal, d = 4.04 Å
- Order of reflection, n = 2
Formula:
The angle of reflection for X-rays in a crystal can be calculated using Bragg's Law:
nλ = 2d sinθ
Calculation:
- Substituting the given values into the formula:
2 * 1.54 Å = 2 * 4.04 Å * sinθ
3.08 Å = 8.08 Å * sinθ
sinθ = 3.08 Å / 8.08 Å
sinθ = 0.381
- Calculating the angle:
θ = sin⁻¹(0.381)
θ ≈ 22°
Therefore, the angle at which the second order reflection will occur in the X-ray spectrometer is approximately 22°. This corresponds to option 'b) 22°'.
Arrange the following atoms and ions in the increasing order of atomic size Mg, Mg2+, Al, Al3+- a)Al3+ > Al > Mg2+ > Mg
- b)Mg2+ Mg > Al3+> Al
- c)Mg > Mg2+ > Al > Al3+
- d)Mg > Al > Mg2+ > Al3+
- e)Al3+ > Mg2+ > Al > Mg
Correct answer is option 'D'. Can you explain this answer?
Arrange the following atoms and ions in the increasing order of atomic size Mg, Mg2+, Al, Al3+
a)
Al3+ > Al > Mg2+ > Mg
b)
Mg2+ Mg > Al3+> Al
c)
Mg > Mg2+ > Al > Al3+
d)
Mg > Al > Mg2+ > Al3+
e)
Al3+ > Mg2+ > Al > Mg
|
|
William Hernandez answered |
The order of atomic size can be determined by comparing the number of protons in the nucleus and the number of electron shells.
Mg: 12 protons, 3 electron shells
Mg2+: 12 protons, 2 electron shells (loss of 2 electrons)
Al: 13 protons, 3 electron shells
Al3+: 13 protons, 2 electron shells (loss of 3 electrons)
Based on this information, the increasing order of atomic size is:
Mg2+ < al3+="" />< mg="" />< al="" />
Mg: 12 protons, 3 electron shells
Mg2+: 12 protons, 2 electron shells (loss of 2 electrons)
Al: 13 protons, 3 electron shells
Al3+: 13 protons, 2 electron shells (loss of 3 electrons)
Based on this information, the increasing order of atomic size is:
Mg2+ < al3+="" />< mg="" />< al="" />
A chemical solution is a homogeneous mixture of one or more substances called “solutes” in another substance called “solvent”. The concentration of a solution is the amount of solute that is dissolved in a certain amount or solvent or solution.There are different units to represent the concentration of a solution, among them there are the molarity (M), defined as the moles of solute present in 1 L of solution; molality, (m) defined as the moles of solute dissolved in 1 kg of solvent; and percentage by mass (%m), which represents the grams of solute dissolved in 100 g of solution.Q. A bottle of concentrated hydrochloric acid has a mass percent of 37.2% and a density of 1.18 g/mL. What is the molarity of concentrated hydrochloric acid? (You may consult the attachment.)- a)0.0120 M
- b)10.2 M
- c)1.20 M
- d)12.0 M
Correct answer is option 'D'. Can you explain this answer?
A chemical solution is a homogeneous mixture of one or more substances called “solutes” in another substance called “solvent”. The concentration of a solution is the amount of solute that is dissolved in a certain amount or solvent or solution.
There are different units to represent the concentration of a solution, among them there are the molarity (M), defined as the moles of solute present in 1 L of solution; molality, (m) defined as the moles of solute dissolved in 1 kg of solvent; and percentage by mass (%m), which represents the grams of solute dissolved in 100 g of solution.
Q. A bottle of concentrated hydrochloric acid has a mass percent of 37.2% and a density of 1.18 g/mL. What is the molarity of concentrated hydrochloric acid? (You may consult the attachment.)
a)
0.0120 M
b)
10.2 M
c)
1.20 M
d)
12.0 M
|
|
Ethan Brown answered |
Solute(s) dissolved in a solvent.
Which of the following is the weakest base?- a)CH3
- b)H-F
- c)H-Cl
- d)H-Br
- e)H-I
Correct answer is option 'E'. Can you explain this answer?
Which of the following is the weakest base?
a)
CH3
b)
H-F
c)
H-Cl
d)
H-Br
e)
H-I
|
|
Ayesha Joshi answered |
The electronegativity and atomic size of iodine is larger so there is a weaker bond between hydrogen and iodine that makes the electron cloud much lesser than H-F bond. So, H-I is the weakest base; in other words it is the strongest acid.
Which of the following is an example of a fibrous protein?- a)Insulin
- b)Hemoglobin
- c)Collagen
- d)Myoglobin
- e)None of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of a fibrous protein?
a)
Insulin
b)
Hemoglobin
c)
Collagen
d)
Myoglobin
e)
None of these
|
|
Ayesha Joshi answered |
Collagen is an example of a fibrous protein. It is a structural protein found in connective tissues, providing strength and flexibility.
Which of the following statements is true about zwitterions?- a)Zwitterions have a net positive charge.
- b)Zwitterions have a net negative charge.
- c)Zwitterions have both positive and negative charges.
- d)Zwitterions do not exist in biological systems.
- e)None of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements is true about zwitterions?
a)
Zwitterions have a net positive charge.
b)
Zwitterions have a net negative charge.
c)
Zwitterions have both positive and negative charges.
d)
Zwitterions do not exist in biological systems.
e)
None of these
|
|
Luna Howard answered |
Zwitterions are molecules or ions that contain both positive and negative charges. They are typically found in biological systems and are important for various biochemical processes. Let's discuss each statement to understand why option C is the correct answer.
a) Zwitterions have a net positive charge.
This statement is not true. Zwitterions have both positive and negative charges, so their overall charge is neutral or zero. The positive and negative charges cancel each other out, resulting in a net charge of zero.
b) Zwitterions have a net negative charge.
This statement is also not true. As mentioned earlier, zwitterions have both positive and negative charges, so their overall charge is neutral or zero. Therefore, they do not have a net negative charge.
c) Zwitterions have both positive and negative charges.
This statement is true. Zwitterions contain both positive and negative charges within the same molecule or ion. They usually have an amino group (-NH3+) with a positive charge and a carboxyl group (-COO-) with a negative charge. This unique combination of charges allows zwitterions to exist in a neutral state.
d) Zwitterions do not exist in biological systems.
This statement is not true. Zwitterions are commonly found in biological systems. For example, amino acids, which are the building blocks of proteins, exist as zwitterions at physiological pH. The presence of zwitterions is essential for the formation and stability of proteins, as well as for various biological processes.
e) None of these
This option is incorrect because option C, which states that zwitterions have both positive and negative charges, is the correct answer.
In summary, zwitterions are molecules or ions that have both positive and negative charges, making their overall charge neutral. They are present in biological systems and play important roles in various biochemical processes.
a) Zwitterions have a net positive charge.
This statement is not true. Zwitterions have both positive and negative charges, so their overall charge is neutral or zero. The positive and negative charges cancel each other out, resulting in a net charge of zero.
b) Zwitterions have a net negative charge.
This statement is also not true. As mentioned earlier, zwitterions have both positive and negative charges, so their overall charge is neutral or zero. Therefore, they do not have a net negative charge.
c) Zwitterions have both positive and negative charges.
This statement is true. Zwitterions contain both positive and negative charges within the same molecule or ion. They usually have an amino group (-NH3+) with a positive charge and a carboxyl group (-COO-) with a negative charge. This unique combination of charges allows zwitterions to exist in a neutral state.
d) Zwitterions do not exist in biological systems.
This statement is not true. Zwitterions are commonly found in biological systems. For example, amino acids, which are the building blocks of proteins, exist as zwitterions at physiological pH. The presence of zwitterions is essential for the formation and stability of proteins, as well as for various biological processes.
e) None of these
This option is incorrect because option C, which states that zwitterions have both positive and negative charges, is the correct answer.
In summary, zwitterions are molecules or ions that have both positive and negative charges, making their overall charge neutral. They are present in biological systems and play important roles in various biochemical processes.
Slater's rule is used to calculate the value of- a)Screening constant
- b)Electron affinity
- c)Ionisation energy
- d)Effective nuclear charge
- e)Both a and d
Correct answer is option 'E'. Can you explain this answer?
Slater's rule is used to calculate the value of
a)
Screening constant
b)
Electron affinity
c)
Ionisation energy
d)
Effective nuclear charge
e)
Both a and d
|
|
Ayesha Joshi answered |
The value of screening constant (S) and effective nuclear charge (Z*) can be calculated using Slater's rule. Effective charge (Z*) = Z – S (where Z- atomic number and S-screening constant).
Which of the following is an example of a tertiary structure in proteins?- a)Alpha-helix
- b)Beta-sheet
- c)Myoglobin
- d)Collagen
- e)None of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of a tertiary structure in proteins?
a)
Alpha-helix
b)
Beta-sheet
c)
Myoglobin
d)
Collagen
e)
None of these
|
|
Paisley Mitchell answered |
Myoglobin is an example of a tertiary structure in proteins.
Explanation:
Tertiary structure refers to the three-dimensional arrangement of the polypeptide chain of a protein. It is a result of interactions between amino acid side chains that are far apart in the primary sequence. The tertiary structure is crucial for determining the overall shape and function of a protein.
Myoglobin is a globular protein found in muscle tissues. It is responsible for binding and storing oxygen in muscle cells. The tertiary structure of myoglobin is primarily maintained by several types of interactions:
1. Hydrophobic Interactions:
Nonpolar side chains tend to cluster together in the interior of the protein, away from water molecules. This hydrophobic core stabilizes the tertiary structure.
2. Hydrogen Bonds:
Polar side chains form hydrogen bonds with each other and with the peptide backbone. These bonds help stabilize the overall structure of the protein.
3. Disulfide Bonds:
Some proteins contain cysteine residues that can form covalent bonds called disulfide bonds. These bonds can contribute to the tertiary structure by connecting different parts of the protein.
4. Ionic Interactions:
Charged side chains can attract or repel each other, contributing to the folding of the protein. These interactions can occur between positively charged (basic) and negatively charged (acidic) amino acid residues.
5. Van der Waals Forces:
Weak attractions between nonpolar side chains can also contribute to the folding of the protein. These forces arise from temporary fluctuations in electron density within atoms.
The tertiary structure of myoglobin results in a compact, globular shape that allows it to efficiently bind and release oxygen. The folding of the polypeptide chain allows for the formation of a hydrophobic pocket where the heme group, a prosthetic group, binds to oxygen.
In summary, myoglobin is an example of a tertiary structure in proteins. Its three-dimensional arrangement is essential for its function as an oxygen-binding protein in muscle tissues.
Explanation:
Tertiary structure refers to the three-dimensional arrangement of the polypeptide chain of a protein. It is a result of interactions between amino acid side chains that are far apart in the primary sequence. The tertiary structure is crucial for determining the overall shape and function of a protein.
Myoglobin is a globular protein found in muscle tissues. It is responsible for binding and storing oxygen in muscle cells. The tertiary structure of myoglobin is primarily maintained by several types of interactions:
1. Hydrophobic Interactions:
Nonpolar side chains tend to cluster together in the interior of the protein, away from water molecules. This hydrophobic core stabilizes the tertiary structure.
2. Hydrogen Bonds:
Polar side chains form hydrogen bonds with each other and with the peptide backbone. These bonds help stabilize the overall structure of the protein.
3. Disulfide Bonds:
Some proteins contain cysteine residues that can form covalent bonds called disulfide bonds. These bonds can contribute to the tertiary structure by connecting different parts of the protein.
4. Ionic Interactions:
Charged side chains can attract or repel each other, contributing to the folding of the protein. These interactions can occur between positively charged (basic) and negatively charged (acidic) amino acid residues.
5. Van der Waals Forces:
Weak attractions between nonpolar side chains can also contribute to the folding of the protein. These forces arise from temporary fluctuations in electron density within atoms.
The tertiary structure of myoglobin results in a compact, globular shape that allows it to efficiently bind and release oxygen. The folding of the polypeptide chain allows for the formation of a hydrophobic pocket where the heme group, a prosthetic group, binds to oxygen.
In summary, myoglobin is an example of a tertiary structure in proteins. Its three-dimensional arrangement is essential for its function as an oxygen-binding protein in muscle tissues.
Which of the following solvents is suitable for SN2 reactions?- a)Ethanol
- b)Water
- c)Acetonitrile
- d)Acetic acid
- e)t-butanol
Correct answer is option 'C'. Can you explain this answer?
Which of the following solvents is suitable for SN2 reactions?
a)
Ethanol
b)
Water
c)
Acetonitrile
d)
Acetic acid
e)
t-butanol
|
|
Ayesha Joshi answered |
Aprotic solvents do not solvate the anions effectively and it is used for SN2 reactions. Acetonitrile is the only aprotic solvent whereas others are polar protic solvents.
Nucleophilic substitution reactions are organic reactions in which an electron-rich substance called a nucleophile reacts with an organic substrate that has an electron-poor carbon atom called an electrophile.
The electrophile has a radical that is a weak base and is substituted by the nucleophile called the leaving group, hence the term nucleophilic substitution.
Nucleophilic substitution can occur via two different mechanisms: SN1, in which the rate determining step is the loss of the leaving group, and SN2, in which the rate-determining step is the nucleophilic attack and the simultaneous loss of the leaving group.
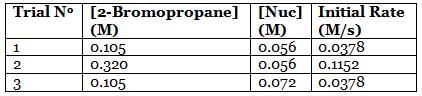
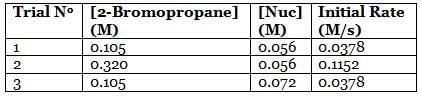
A scientist is studying the nucleophilic substitution reaction between 2-Bromopropane and a novel nucleophile (Nuc), collecting the kinetic data that is shown in the table below:
 Q. The reaction between 2-Bromopropane and the novel nucleophile Nuc proceeds via an ____ mechanism in which the rate law is ____. (You may consult the attachment.)
Q. The reaction between 2-Bromopropane and the novel nucleophile Nuc proceeds via an ____ mechanism in which the rate law is ____. (You may consult the attachment.)- a)SN1,r = k⋅[2 − Bromopropane]
- b)SN2, r = k⋅[2−Bromopropane]⋅[Nuc]
- c)SN2, r = k⋅[2 − Bromopropane]
- d)SN1, r = k⋅[2 − Bromopropane]⋅[Nuc]
Correct answer is option 'A'. Can you explain this answer?
Nucleophilic substitution reactions are organic reactions in which an electron-rich substance called a nucleophile reacts with an organic substrate that has an electron-poor carbon atom called an electrophile.
The electrophile has a radical that is a weak base and is substituted by the nucleophile called the leaving group, hence the term nucleophilic substitution.
Nucleophilic substitution can occur via two different mechanisms: SN1, in which the rate determining step is the loss of the leaving group, and SN2, in which the rate-determining step is the nucleophilic attack and the simultaneous loss of the leaving group.
A scientist is studying the nucleophilic substitution reaction between 2-Bromopropane and a novel nucleophile (Nuc), collecting the kinetic data that is shown in the table below:

The electrophile has a radical that is a weak base and is substituted by the nucleophile called the leaving group, hence the term nucleophilic substitution.
Nucleophilic substitution can occur via two different mechanisms: SN1, in which the rate determining step is the loss of the leaving group, and SN2, in which the rate-determining step is the nucleophilic attack and the simultaneous loss of the leaving group.
A scientist is studying the nucleophilic substitution reaction between 2-Bromopropane and a novel nucleophile (Nuc), collecting the kinetic data that is shown in the table below:

Q. The reaction between 2-Bromopropane and the novel nucleophile Nuc proceeds via an ____ mechanism in which the rate law is ____. (You may consult the attachment.)
a)
SN1,r = k⋅[2 − Bromopropane]
b)
SN2, r = k⋅[2−Bromopropane]⋅[Nuc]
c)
SN2, r = k⋅[2 − Bromopropane]
d)
SN1, r = k⋅[2 − Bromopropane]⋅[Nuc]

|
Orion Classes answered |
To find out the mechanism and the rate law of the reaction, the reaction order with respect to each reactant needs to be determined using the method of initial rates. For 2-Bromopropane, trials 1 and 2 are selected and the reaction order is found in the following way:
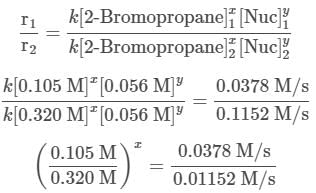
The two fractions are equivalent, hence x = 1. Now, for Nuc, trials 1 and 3 are selected, where the concentration of Nuc is kept constant, and the reaction order is found in the following way:
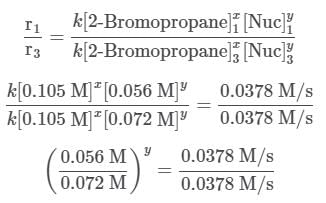
Since needs to be equal to 1, it means that y = 0. These reaction orders show that the reaction proceeds through an SN1 mechanism in which the rate law is r = k⋅[2 − Bromopropane]
needs to be equal to 1, it means that y = 0. These reaction orders show that the reaction proceeds through an SN1 mechanism in which the rate law is r = k⋅[2 − Bromopropane]

The two fractions are equivalent, hence x = 1. Now, for Nuc, trials 1 and 3 are selected, where the concentration of Nuc is kept constant, and the reaction order is found in the following way:

Since
 needs to be equal to 1, it means that y = 0. These reaction orders show that the reaction proceeds through an SN1 mechanism in which the rate law is r = k⋅[2 − Bromopropane]
needs to be equal to 1, it means that y = 0. These reaction orders show that the reaction proceeds through an SN1 mechanism in which the rate law is r = k⋅[2 − Bromopropane]Which of the following groups has –I effect?- a)-CH3
- b)-C2 H5
- c)-C(CH3)3
- d)–C6 H5
- e)Both a and c
Correct answer is option 'D'. Can you explain this answer?
Which of the following groups has –I effect?
a)
-CH3
b)
-C2 H5
c)
-C(CH3)3
d)
–C6 H5
e)
Both a and c

|
Orion Classes answered |
The polarisation of the bond is due to electron withdrawing or electron donating effect of adjacent atoms or groups. Such a type of electron displacement along a carbon chain is called Inductive effect.
Eg: C –>--- C –>--- C –>--- C6 H5
The electron withdrawing nature of groups or atoms is called negative inductive effect. C6H5 is the only group, which has –I effect. Since --C6 H5 is electronwithdrawing group, it pulls up the electrons towards itself. Thereby it creates a partial positive charge in adjacent carbon atoms and partial negative charge in phenyl group (C6 H5).
Eg: C –>--- C –>--- C –>--- C6 H5
The electron withdrawing nature of groups or atoms is called negative inductive effect. C6H5 is the only group, which has –I effect. Since --C6 H5 is electronwithdrawing group, it pulls up the electrons towards itself. Thereby it creates a partial positive charge in adjacent carbon atoms and partial negative charge in phenyl group (C6 H5).
Compound A reacts by first order kinetics. The rate constant of the reaction is 0.45 sec-1. Calculatethe half-life of the compound A in the reaction.- a)4.62 seconds
- b)3.08 seconds
- c)1.54 seconds
- d)2.25 seconds
- e)0.9 seconds
Correct answer is option 'C'. Can you explain this answer?
Compound A reacts by first order kinetics. The rate constant of the reaction is 0.45 sec-1. Calculatethe half-life of the compound A in the reaction.
a)
4.62 seconds
b)
3.08 seconds
c)
1.54 seconds
d)
2.25 seconds
e)
0.9 seconds
|
|
David Baker answered |
Explanation:
The rate of a first-order reaction follows the equation: ln([A]t/[A]0) = -kt, where [A]t is the concentration of compound A at time t, [A]0 is the initial concentration of compound A, k is the rate constant, and t is the time.
To find the half-life of a first-order reaction, we need to find the time it takes for the concentration of compound A to decrease by half.
We can rearrange the equation for a first-order reaction to solve for t:
ln([A]t/[A]0) = -kt
ln(1/2) = -k*t1/2
-ln(2) = -k*t1/2
Since ln(2) is a constant value, we can substitute the given rate constant (k = 0.45 sec^-1) into the equation and solve for t1/2:
-ln(2) = -0.45*t1/2
t1/2 = ln(2)/0.45
Using the value of ln(2) = 0.6931, we can calculate the half-life:
t1/2 = 0.6931/0.45
t1/2 ≈ 1.54 seconds
Therefore, the correct answer is option C) 1.54 seconds.
The rate of a first-order reaction follows the equation: ln([A]t/[A]0) = -kt, where [A]t is the concentration of compound A at time t, [A]0 is the initial concentration of compound A, k is the rate constant, and t is the time.
To find the half-life of a first-order reaction, we need to find the time it takes for the concentration of compound A to decrease by half.
We can rearrange the equation for a first-order reaction to solve for t:
ln([A]t/[A]0) = -kt
ln(1/2) = -k*t1/2
-ln(2) = -k*t1/2
Since ln(2) is a constant value, we can substitute the given rate constant (k = 0.45 sec^-1) into the equation and solve for t1/2:
-ln(2) = -0.45*t1/2
t1/2 = ln(2)/0.45
Using the value of ln(2) = 0.6931, we can calculate the half-life:
t1/2 = 0.6931/0.45
t1/2 ≈ 1.54 seconds
Therefore, the correct answer is option C) 1.54 seconds.
The one which is most commonly used as a detection of developed colorless chromatogram spots in T.L.C plate is- a)Iodine
- b)Phosphorus
- c)Water
- d)Copper salts
- e)Ammonia
Correct answer is option 'A'. Can you explain this answer?
The one which is most commonly used as a detection of developed colorless chromatogram spots in T.L.C plate is
a)
Iodine
b)
Phosphorus
c)
Water
d)
Copper salts
e)
Ammonia
|
|
Amelia Taylor answered |
Explanation:
Iodine
- Iodine is the most commonly used reagent for the detection of developed colorless chromatogram spots in TLC (Thin Layer Chromatography) plates.
- When the TLC plate is exposed to iodine vapor, the colorless spots on the plate will become visible due to the formation of colored complexes between iodine and the compounds present in the spots.
- These complexes can be easily observed as brown or purple spots against the background of the TLC plate.
- Iodine is particularly useful for detecting a wide range of compounds, making it a versatile choice for TLC analysis.
- The iodine vapor detection method is simple, quick, and efficient, making it a popular choice in laboratories for TLC analysis.
In conclusion, iodine is the go-to reagent for visualizing colorless spots on TLC plates, making it an essential tool for identifying and analyzing compounds in chromatography experiments.
Iodine
- Iodine is the most commonly used reagent for the detection of developed colorless chromatogram spots in TLC (Thin Layer Chromatography) plates.
- When the TLC plate is exposed to iodine vapor, the colorless spots on the plate will become visible due to the formation of colored complexes between iodine and the compounds present in the spots.
- These complexes can be easily observed as brown or purple spots against the background of the TLC plate.
- Iodine is particularly useful for detecting a wide range of compounds, making it a versatile choice for TLC analysis.
- The iodine vapor detection method is simple, quick, and efficient, making it a popular choice in laboratories for TLC analysis.
In conclusion, iodine is the go-to reagent for visualizing colorless spots on TLC plates, making it an essential tool for identifying and analyzing compounds in chromatography experiments.
Systematic error is also called- a)Experimental error
- b)Random error
- c)Indeterminate error
- d)Determinate error
- e)None of these.
Correct answer is option 'D'. Can you explain this answer?
Systematic error is also called
a)
Experimental error
b)
Random error
c)
Indeterminate error
d)
Determinate error
e)
None of these.
|
|
Ayesha Joshi answered |
Determinate errors, also known as systematic errors, are errors that are known and controllable. These errors can arise from factors such as instrumental limitations or personal mistakes. On the other hand, indeterminate errors, also called random errors, are unknown errors that cannot be precisely identified or controlled. An example of an indeterminate error could be the fluctuation in room temperature during an experiment.
Which of the following statements is true about the secondary structure of proteins?- a)The alpha-helix is an example of a secondary structure.
- b)Secondary structure is determined by the linear sequence of amino acids.
- c)Secondary structure involves the formation of disulfide bonds.
- d)The secondary structure determines the three-dimensional folding of a protein.
- e)None of these
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements is true about the secondary structure of proteins?
a)
The alpha-helix is an example of a secondary structure.
b)
Secondary structure is determined by the linear sequence of amino acids.
c)
Secondary structure involves the formation of disulfide bonds.
d)
The secondary structure determines the three-dimensional folding of a protein.
e)
None of these
|
|
Ayesha Joshi answered |
The alpha-helix is a common secondary structure in proteins, characterized by a right-handed coiled arrangement of the polypeptide chain. Secondary structure is primarily determined by hydrogen bonding between the backbone atoms of the amino acids.
The base peak in a mass spectrum is- a)The peak set to 100% relative intensity
- b)The peak set to 0% relative intensity
- c)The peak corresponding to the parent ion
- d)The highest mass peak
- e)The lowest mass peak
Correct answer is option 'A'. Can you explain this answer?
The base peak in a mass spectrum is
a)
The peak set to 100% relative intensity
b)
The peak set to 0% relative intensity
c)
The peak corresponding to the parent ion
d)
The highest mass peak
e)
The lowest mass peak
|
|
Ayesha Joshi answered |
The most intense peak is called as base peak. It usually corresponds to the molecular ion only, if the spectra are recorded at low ionization energy.
The branch of physics that studies the motion of a body without taking into account the causes of that motion is called kinematics. According to kinematics, the position and velocity of a moving body is described by the following equations:
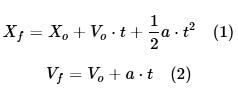
Where Xo and Xf are the initial and final positions of the body, Vo and Vf are the initial and final velocities of the body, a is the acceleration of the body, and t is the time.
According to classical mechanics, developed by Isaac Newton, the product of the mass and the acceleration of a body can be described by the following equation (Newton’s second law):
m⋅a = ΣF
Where m is the mass, a is the acceleration, and ΣF is the sum of all forces acting on the body.
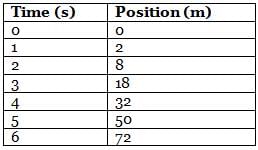
The following table shows the position at different times of a 80-kg hospital bed with a 40-kg patient on top of it that is being pushed from rest by a doctor on a rough floor that exerts a constant friction force of 600 N.
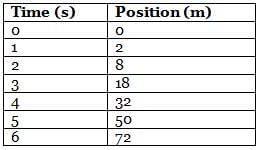 Q. What is the force exerted by the doctor on the bed in the attachment?
Q. What is the force exerted by the doctor on the bed in the attachment?- a)920 N
- b)480 N
- c)1080 N
- d)760 N
Correct answer is option 'C'. Can you explain this answer?
The branch of physics that studies the motion of a body without taking into account the causes of that motion is called kinematics. According to kinematics, the position and velocity of a moving body is described by the following equations:
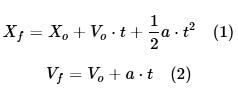
Where Xo and Xf are the initial and final positions of the body, Vo and Vf are the initial and final velocities of the body, a is the acceleration of the body, and t is the time.
According to classical mechanics, developed by Isaac Newton, the product of the mass and the acceleration of a body can be described by the following equation (Newton’s second law):
m⋅a = ΣF
Where m is the mass, a is the acceleration, and ΣF is the sum of all forces acting on the body.
The following table shows the position at different times of a 80-kg hospital bed with a 40-kg patient on top of it that is being pushed from rest by a doctor on a rough floor that exerts a constant friction force of 600 N.

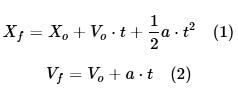
Where Xo and Xf are the initial and final positions of the body, Vo and Vf are the initial and final velocities of the body, a is the acceleration of the body, and t is the time.
According to classical mechanics, developed by Isaac Newton, the product of the mass and the acceleration of a body can be described by the following equation (Newton’s second law):
m⋅a = ΣF
Where m is the mass, a is the acceleration, and ΣF is the sum of all forces acting on the body.
The following table shows the position at different times of a 80-kg hospital bed with a 40-kg patient on top of it that is being pushed from rest by a doctor on a rough floor that exerts a constant friction force of 600 N.

Q. What is the force exerted by the doctor on the bed in the attachment?
a)
920 N
b)
480 N
c)
1080 N
d)
760 N
|
|
Ayesha Joshi answered |
The acceleration can be found by solving the motion equation, considering the initial velocity and position to be zero, for any time. For example, for t = 2 s:
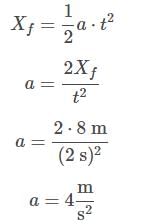
Then, the force exerted by the doctor, Fd, can be found by applying Newton’s second law on the bed:
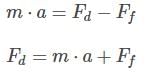
Plugging in the appropriate values, and considering the mass to be equal to the sum of the mass of the bed and the mass of the patient:
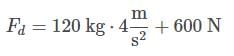
Fd = 1080 N
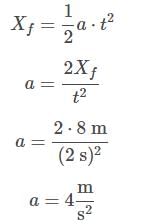
Then, the force exerted by the doctor, Fd, can be found by applying Newton’s second law on the bed:
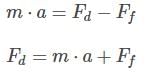
Plugging in the appropriate values, and considering the mass to be equal to the sum of the mass of the bed and the mass of the patient:
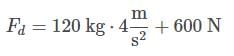
Fd = 1080 N
The branch of physics that studies the motion of a body without taking into account the causes of that motion is called kinematics. According to kinematics, the position and velocity of a moving body is described by the following equations:
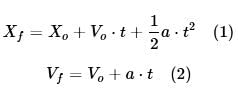
Where Xo and Xf are the initial and final positions of the body, Vo and Vf are the initial and final velocities of the body, a is the acceleration of the body, and t is the time.
According to classical mechanics, developed by Isaac Newton, the product of the mass and the acceleration of a body can be described by the following equation (Newton’s second law):
m⋅a = ΣF
Where m is the mass, a is the acceleration, and ΣF is the sum of all forces acting on the body.
The following table shows the position at different times of a 80-kg hospital bed with a 40-kg patient on top of it that is being pushed from rest by a doctor on a rough floor that exerts a constant friction force of 600 N.
 Q. What is the acceleration of the hospital bed in the attachment?
Q. What is the acceleration of the hospital bed in the attachment?- a)
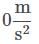
- b)
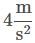
- c)
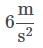
- d)
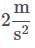
Correct answer is option 'B'. Can you explain this answer?
The branch of physics that studies the motion of a body without taking into account the causes of that motion is called kinematics. According to kinematics, the position and velocity of a moving body is described by the following equations:
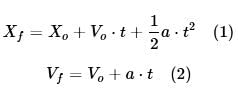
Where Xo and Xf are the initial and final positions of the body, Vo and Vf are the initial and final velocities of the body, a is the acceleration of the body, and t is the time.
According to classical mechanics, developed by Isaac Newton, the product of the mass and the acceleration of a body can be described by the following equation (Newton’s second law):
m⋅a = ΣF
Where m is the mass, a is the acceleration, and ΣF is the sum of all forces acting on the body.
The following table shows the position at different times of a 80-kg hospital bed with a 40-kg patient on top of it that is being pushed from rest by a doctor on a rough floor that exerts a constant friction force of 600 N.

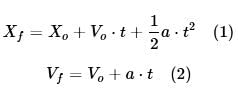
Where Xo and Xf are the initial and final positions of the body, Vo and Vf are the initial and final velocities of the body, a is the acceleration of the body, and t is the time.
According to classical mechanics, developed by Isaac Newton, the product of the mass and the acceleration of a body can be described by the following equation (Newton’s second law):
m⋅a = ΣF
Where m is the mass, a is the acceleration, and ΣF is the sum of all forces acting on the body.
The following table shows the position at different times of a 80-kg hospital bed with a 40-kg patient on top of it that is being pushed from rest by a doctor on a rough floor that exerts a constant friction force of 600 N.

Q. What is the acceleration of the hospital bed in the attachment?
a)
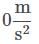
b)
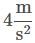
c)
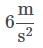
d)
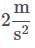
|
|
Ayesha Joshi answered |
The acceleration can be found by solving the motion equation, considering the initial velocity and position to be zero, for any time. For example, for t = 2:
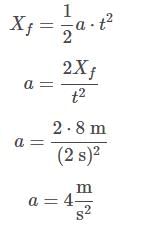
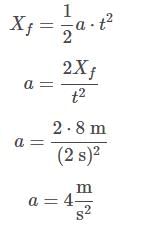
An example for interpolation error is- a)Incorrect identification of indicator's color change in titration
- b)Guessing the correct value between two calibrated marks on the metre scale
- c)Zero setting of the needle in analog display
- d)Calibration of measured instrument
- e)None of these
Correct answer is option 'B'. Can you explain this answer?
An example for interpolation error is
a)
Incorrect identification of indicator's color change in titration
b)
Guessing the correct value between two calibrated marks on the metre scale
c)
Zero setting of the needle in analog display
d)
Calibration of measured instrument
e)
None of these
|
|
Ayesha Joshi answered |
Guessing the correct value between two calibrated marks on the metre scale is an example for interpolation error. It is one of the two types of human or personal errors. Incorrect identification of indicator's color change in titration – Operative error. Zero setting of the needle in analog display – static error. Calibration of measured instrument - Instrument error.
In which of the following processes, is the process always non-feasible?- a)ΔH > 0, ΔS > 0
- b)ΔH < 0, ΔS > 0
- c)ΔH > 0, ΔS < 0
- d)ΔH < 0, ΔS < 0
- e)ΔH = 0, ΔS = 0
Correct answer is option 'C'. Can you explain this answer?
In which of the following processes, is the process always non-feasible?
a)
ΔH > 0, ΔS > 0
b)
ΔH < 0, ΔS > 0
c)
ΔH > 0, ΔS < 0
d)
ΔH < 0, ΔS < 0
e)
ΔH = 0, ΔS = 0
|
|
Ayesha Joshi answered |
For a non-spontaneous or non-feasible process, ΔH > 0 and ΔS < 0. For a spontaneous or irreversible reaction, ΔH < 0 and ΔS > 0. For an equilibrium or reversible process, ΔH = 0 and ΔS = 0.
If 3.21 g of silver nitrate reacts with an excess of sodium sulfate, what mass of silver sulfate will be produced?
(MM silver nitrate = 169.87 g/mol; MM sodium sulfate = 142.04 g/mol; MM silver sulfate = 311.799 g/mol; MM sodium nitrate = 84.9947 g/mol)11.8 g- a)11.8 g
- b)1.61 g
- c)5.89 g
- d)2.95 g
Correct answer is option 'D'. Can you explain this answer?
If 3.21 g of silver nitrate reacts with an excess of sodium sulfate, what mass of silver sulfate will be produced?
(MM silver nitrate = 169.87 g/mol; MM sodium sulfate = 142.04 g/mol; MM silver sulfate = 311.799 g/mol; MM sodium nitrate = 84.9947 g/mol)11.8 g
(MM silver nitrate = 169.87 g/mol; MM sodium sulfate = 142.04 g/mol; MM silver sulfate = 311.799 g/mol; MM sodium nitrate = 84.9947 g/mol)11.8 g
a)
11.8 g
b)
1.61 g
c)
5.89 g
d)
2.95 g
|
|
Ayesha Joshi answered |
The balanced chemical equation between silver nitrate and sodium sulfate is the following:

To calculate the mass of silver sulfate produced, the following conversion factors are applied:


To calculate the mass of silver sulfate produced, the following conversion factors are applied:

Which of the following are nucleophiles?- a)H+
- b)H3O+
- c)CO2
- d)AlCl3
- e)NH3
Correct answer is option 'E'. Can you explain this answer?
Which of the following are nucleophiles?
a)
H+
b)
H3O+
c)
CO2
d)
AlCl3
e)
NH3
|
|
Ayesha Joshi answered |
In the case of ammonia (NH3), it has a lone pair of electrons on the nitrogen atom, which allows it to act as a nucleophile by donating that lone pair. On the other hand, the other molecules mentioned are not electron pair donors like ammonia. Instead, they are often classified as electrophiles. Electrophiles are molecules or ions that can accept an electron pair from a nucleophile to form new bonds. These molecules have electron-deficient regions or positively charged atoms that can attract and accept an electron pair. Therefore, except for ammonia, the rest of the molecules mentioned can be considered electrophiles. They have regions or atoms that are capable of accepting an electron pair in a chemical reaction.
The IUPAC notation for representing an atom is 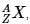 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:
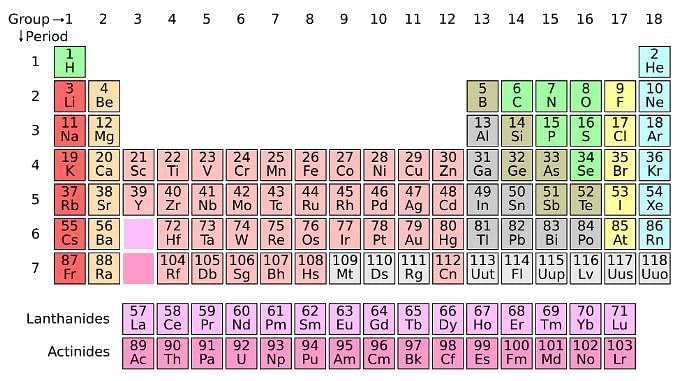 The location of an element in the periodic table influences its properties. Among the most important periodic trends are ionization energy, which increases from left to right and from bottom to top; atomic radius, which increases from right to left and from top to bottom; and electron affinity, which increases from left to right and from bottom to top.Q. Which of the following elements has the smallest ionization energy? (See attachment.)
The location of an element in the periodic table influences its properties. Among the most important periodic trends are ionization energy, which increases from left to right and from bottom to top; atomic radius, which increases from right to left and from top to bottom; and electron affinity, which increases from left to right and from bottom to top.Q. Which of the following elements has the smallest ionization energy? (See attachment.)- a)Na
- b)Si
- c)Sr
- d)I
Correct answer is option 'C'. Can you explain this answer?
The IUPAC notation for representing an atom is 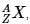 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:

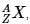 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:

The location of an element in the periodic table influences its properties. Among the most important periodic trends are ionization energy, which increases from left to right and from bottom to top; atomic radius, which increases from right to left and from top to bottom; and electron affinity, which increases from left to right and from bottom to top.
Q. Which of the following elements has the smallest ionization energy? (See attachment.)
a)
Na
b)
Si
c)
Sr
d)
I
|
|
Ayesha Joshi answered |
From the atoms in the options, the one that is located further down in the periodic table is Sr. Na is located more to the left, but when deciding between Na and Sr, the highest principal energy level of Sr means that its electrons are located farther away from the nucleus and hence are easier to remove from the valence layer, making it the atom with the smallest ionization energy.
Identify the unit of concentration of the solution (NA)/(Kg of solvent).- a)Molarity
- b)Molality
- c)Normality
- d)Mole fraction
- e)ppm
Correct answer is option 'B'. Can you explain this answer?
Identify the unit of concentration of the solution (NA)/(Kg of solvent).
a)
Molarity
b)
Molality
c)
Normality
d)
Mole fraction
e)
ppm
|
|
Ayesha Joshi answered |
Molarity (MA) = nA/ volume in litres.
Normality = Gram equivalent of A/Volume in litres of solution.
Mole fraction (χi) = ni/ (n1+n2+n3....).
Parts per million (ppm) = (Mass of A/Total mass) x 106
Normality = Gram equivalent of A/Volume in litres of solution.
Mole fraction (χi) = ni/ (n1+n2+n3....).
Parts per million (ppm) = (Mass of A/Total mass) x 106
Which of the following is responsible for the secondary structure of proteins?- a)Hydrogen bonds between amino acid side chains
- b)Hydrophobic interactions
- c)Peptide bonds between amino acids
- d)Disulfide bonds between cysteine residues
- e)None of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following is responsible for the secondary structure of proteins?
a)
Hydrogen bonds between amino acid side chains
b)
Hydrophobic interactions
c)
Peptide bonds between amino acids
d)
Disulfide bonds between cysteine residues
e)
None of these
|
|
Ayesha Joshi answered |
The secondary structure of proteins is primarily determined by the hydrogen bonding between the carbonyl oxygen of one amino acid and the amino hydrogen of another amino acid along the polypeptide backbone, which is facilitated by the peptide bonds.
Which of the following amino acids is negatively charged at physiological pH?- a)Aspartic acid
- b)Histidine
- c)Arginine
- d)Glutamine
- e)None of these
Correct answer is option 'A'. Can you explain this answer?
Which of the following amino acids is negatively charged at physiological pH?
a)
Aspartic acid
b)
Histidine
c)
Arginine
d)
Glutamine
e)
None of these
|
|
Ayesha Joshi answered |
Aspartic acid has a carboxyl group (COOH) in its side chain, which can ionize and become negatively charged at physiological pH.
DNA (deoxyribonucleic acid) is the information-storing biomolecule of the cell. The process by which the information stored in DNA can be converted into proteins can be broken down into three subprocesses:Replication: In this process, a parent DNA strand is paired with its complementary strand. Adenine is paired with thymine, cytosine is paired with guanine, and vice-versa. In this way the information stored in DNA is preserved.
Transcription: In this process, DNA is separated and the complementary strand is copied into mRNA. Adenine is paired with uracil, cytosine is paired with guanine, and vice-versa. Translation: In this process, tRNA recognizes sequences of three bases in RNA called codons and transfers an amino acid into the growing protein chain that starts with the codon that codes for methionine. The codons are shown in the image below and are read starting from the center:
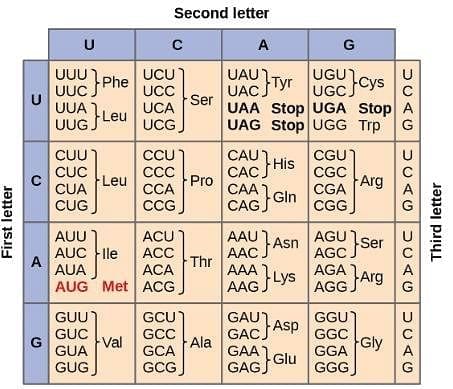 Q. Using the attachment, how many amino acids would be located on the polypeptide chain made from the sequence ATTATGCGTCAATGAATAAGACTG?
Q. Using the attachment, how many amino acids would be located on the polypeptide chain made from the sequence ATTATGCGTCAATGAATAAGACTG?- a)7
- b)8
- c)4
- d)3
Correct answer is option 'D'. Can you explain this answer?
DNA (deoxyribonucleic acid) is the information-storing biomolecule of the cell. The process by which the information stored in DNA can be converted into proteins can be broken down into three subprocesses:
Replication: In this process, a parent DNA strand is paired with its complementary strand. Adenine is paired with thymine, cytosine is paired with guanine, and vice-versa. In this way the information stored in DNA is preserved.
Transcription: In this process, DNA is separated and the complementary strand is copied into mRNA. Adenine is paired with uracil, cytosine is paired with guanine, and vice-versa. Translation: In this process, tRNA recognizes sequences of three bases in RNA called codons and transfers an amino acid into the growing protein chain that starts with the codon that codes for methionine. The codons are shown in the image below and are read starting from the center:

Transcription: In this process, DNA is separated and the complementary strand is copied into mRNA. Adenine is paired with uracil, cytosine is paired with guanine, and vice-versa. Translation: In this process, tRNA recognizes sequences of three bases in RNA called codons and transfers an amino acid into the growing protein chain that starts with the codon that codes for methionine. The codons are shown in the image below and are read starting from the center:

Q. Using the attachment, how many amino acids would be located on the polypeptide chain made from the sequence ATTATGCGTCAATGAATAAGACTG?
a)
7
b)
8
c)
4
d)
3
|
|
Ayesha Joshi answered |
The complementary DNA chain for this sequence would be TAATACGCAGTTACTTATTCTGAC, and the mRNA sequence associated with this chain would be AUUAUGCGUCAAUGAAUAAGACUG. This sequence starts at AUG (Which codes for Met and is a START codon) and codes for Met-Arg-Gln. After Gln, the next codon is UGA, which is a STOP codon, signaling the end of the polypeptide chain and its release from the ribosome. This means that the polypeptide chain is 3 amino acids long.
A chemical solution is a homogeneous mixture of one or more substances called “solutes” in another substance called “solvent”. The concentration of a solution is the amount of solute that is dissolved in a certain amount or solvent or solution.There are different units to represent the concentration of a solution, among them there are the molarity (M), defined as the moles of solute present in 1 L of solution; molality, (m) defined as the moles of solute dissolved in 1 kg of solvent; and percentage by mass (%m), which represents the grams of solute dissolved in 100 g of solution.Q. What mass of KBr is needed to prepare 250 mL of a 0.200 M solution of KBr?MM KBr = 119.002 g/mol (See attachment.)- a)0.420 g
- b)23.8 g
- c)5.95 g
- d)5.95 kg
Correct answer is option 'C'. Can you explain this answer?
A chemical solution is a homogeneous mixture of one or more substances called “solutes” in another substance called “solvent”. The concentration of a solution is the amount of solute that is dissolved in a certain amount or solvent or solution.
There are different units to represent the concentration of a solution, among them there are the molarity (M), defined as the moles of solute present in 1 L of solution; molality, (m) defined as the moles of solute dissolved in 1 kg of solvent; and percentage by mass (%m), which represents the grams of solute dissolved in 100 g of solution.
Q. What mass of KBr is needed to prepare 250 mL of a 0.200 M solution of KBr?MM KBr = 119.002 g/mol (See attachment.)
a)
0.420 g
b)
23.8 g
c)
5.95 g
d)
5.95 kg
|
|
Ayesha Joshi answered |
In order to find the mass of KBr needed, the following conversion factors can be applied to go from mL of solution to mass of KBr:
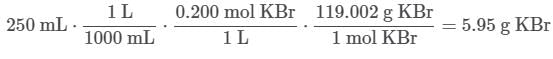
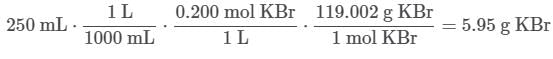
An ideal gas can be defined thermodynamically, when,
I. PV = constant
II. (∂U/∂V)p = 0
III. (∂U/∂V)T = 0- a)I only
- b)I & II
- c)I & III
- d)II & III
- e)II
Correct answer is option 'C'. Can you explain this answer?
An ideal gas can be defined thermodynamically, when,
I. PV = constant
II. (∂U/∂V)p = 0
III. (∂U/∂V)T = 0
I. PV = constant
II. (∂U/∂V)p = 0
III. (∂U/∂V)T = 0
a)
I only
b)
I & II
c)
I & III
d)
II & III
e)
II
|
|
Ayesha Joshi answered |
For an ideal gas, PV = constant, at constant temperature. The internal energy of a given quantity of an ideal gas at a constant temperature is independent of its volume, thus (∂U/∂V)T = 0.
Which of the following amino acids is classified as basic?- a)Alanine
- b)Serine
- c)Lysine
- d)Phenylalanine
- e)None of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following amino acids is classified as basic?
a)
Alanine
b)
Serine
c)
Lysine
d)
Phenylalanine
e)
None of these
|
|
Ayesha Joshi answered |
Lysine is classified as a basic amino acid due to the presence of an amino group (NH2) in its side chain. It can accept protons and become positively charged at physiological pH.
Which one of the following molecules is held together by dative bond?- a)AlBr3
- b)NaCl
- c)Al2Cl6
- d)C2H6
- e)H2O2
Correct answer is option 'C'. Can you explain this answer?
Which one of the following molecules is held together by dative bond?
a)
AlBr3
b)
NaCl
c)
Al2Cl6
d)
C2H6
e)
H2O2
|
|
Ayesha Joshi answered |
In Al2 Cl6 lone pairs of electron from chlorine are donated to electron deficient aluminium in such a way that it is held together by dative bond, where aluminium acts as an electron acceptor and chlorine acts as an electron donor. AlBr3 is a neutral ionic molecule. NaCl- ionic bond, C2 H6 - covalent bond,H2 O2– Hydrogen bond.
Predict whether the following half-cell reactions are Oxidation or Reduction.
I) Cu2+ (aq) + 2e- → Cu(s)
II) Cu(s) → Cu2+ (aq) + 2e-- a)I-Oxidation reaction, II- Reduction reaction
- b)I-Reduction reaction, II- Oxidation reaction
- c)Both I and II are Oxidation reactions
- d)Both I and II are Reduction reactions
- e)None of the above
Correct answer is option 'B'. Can you explain this answer?
Predict whether the following half-cell reactions are Oxidation or Reduction.
I) Cu2+ (aq) + 2e- → Cu(s)
II) Cu(s) → Cu2+ (aq) + 2e-
I) Cu2+ (aq) + 2e- → Cu(s)
II) Cu(s) → Cu2+ (aq) + 2e-
a)
I-Oxidation reaction, II- Reduction reaction
b)
I-Reduction reaction, II- Oxidation reaction
c)
Both I and II are Oxidation reactions
d)
Both I and II are Reduction reactions
e)
None of the above
|
|
Ayesha Joshi answered |
A reduction reaction occurs when an atom or ion accepts an electron, while an oxidation reaction occurs when an atom or ion releases an electron. In the first mentioned reaction, the Cu2+ ion accepts two electrons, indicating a reduction process. Conversely, in the second reaction, the Cu atom releases two electrons, indicating an oxidation process.
The scale which is based on an empirical relation between the energy of a bond and the electronegativities of bonded atoms is- a)Pauling scale
- b)Mulliken's scale
- c)Sanderson's scale
- d)Alfred and Rochow's scale
- e)Both a and b
Correct answer is option 'A'. Can you explain this answer?
The scale which is based on an empirical relation between the energy of a bond and the electronegativities of bonded atoms is
a)
Pauling scale
b)
Mulliken's scale
c)
Sanderson's scale
d)
Alfred and Rochow's scale
e)
Both a and b
|
|
Ayesha Joshi answered |
- The Pauling electronegativity scale is indeed based on an empirical relation between the energy of a bond and the electronegativities of the atoms involved. Linus Pauling developed this scale, which assigns electronegativity values to elements. It provides a measure of an element's ability to attract electron density towards itself when it forms a chemical bond.
- On the other hand, the Mulliken electronegativity scale is based on the ionization energy (the energy required to remove an electron from an atom) and the electron affinity (the energy change when an atom gains an electron) of an atom. This scale, developed by Robert S. Mulliken, associates electronegativity values with elements based on these electronic properties.
- Both the Pauling and Mulliken electronegativity scales aim to quantify the relative ability of atoms to attract electrons in a chemical bond, but they employ different factors and approaches in their calculations.
The relative intensity of signals in proton NMR is related to- a)Chemical shift and magnetic environment of proton
- b)Different number of protons
- c)Number of adjacent atoms containing number of protons
- d)Total number of protons present in the molecule
- e)Coupling constant
Correct answer is option 'D'. Can you explain this answer?
The relative intensity of signals in proton NMR is related to
a)
Chemical shift and magnetic environment of proton
b)
Different number of protons
c)
Number of adjacent atoms containing number of protons
d)
Total number of protons present in the molecule
e)
Coupling constant
|
|
Ayesha Joshi answered |
The intensity of signals in proton NMR is directly proportional to the total number of protons present in the molecule. The number of signals corresponds to the different types of protons present in the molecule. The position of signals reflects the chemical shift and the magnetic environment experienced by the protons. The splitting of signals indicates the presence of adjacent atoms that have a different number of protons.
Which of the following is a Bronsted-Lowry acid?- a)CH2=CH2
- b)CH3NH2
- c)CH3CO2H
- d)CH3COCH3
- e)C6H10
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a Bronsted-Lowry acid?
a)
CH2=CH2
b)
CH3NH2
c)
CH3CO2H
d)
CH3COCH3
e)
C6H10
|
|
Ayesha Joshi answered |
All Bronsted-Lowry acids have the ability to donate a proton. The net charge of an acid may be zero, positive, or negative depending on the specific acid. In the options mentioned, acetic acid (CH3CO2H) is the only molecule that can liberate a proton and act as a proton donor. It donates a proton from its acidic hydrogen atom (H+) when it undergoes a chemical reaction.
Calculate the molarity (M), when 580g of NaClis added to 2L of water.- a)5 M
- b)3 M
- c)2.5 M
- d)80 M
- e)10 M
Correct answer is option 'A'. Can you explain this answer?
Calculate the molarity (M), when 580g of NaClis added to 2L of water.
a)
5 M
b)
3 M
c)
2.5 M
d)
80 M
e)
10 M
|
|
Ayesha Joshi answered |
Molarity (M) = (moles of solute/litres of solution)
Molecular weight of NaCl = 23 + 35= 58 g/mol
Moles of NaCl = 580/58 = 10 mol
Therefore, Molarity (M) = 10 mol/2 L= 5 M
Molecular weight of NaCl = 23 + 35= 58 g/mol
Moles of NaCl = 580/58 = 10 mol
Therefore, Molarity (M) = 10 mol/2 L= 5 M
Which of the following amino acids has a sulfur-containing side chain?- a)Tyrosine
- b)Tryptophan
- c)Cysteine
- d)Aspartic acid
- e)None of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following amino acids has a sulfur-containing side chain?
a)
Tyrosine
b)
Tryptophan
c)
Cysteine
d)
Aspartic acid
e)
None of these
|
|
Ayesha Joshi answered |
Cysteine is the amino acid with a sulfur-containing side chain. It plays a crucial role in the formation of disulfide bonds, contributing to the protein's higher-order structure.
Nucleophilic substitution reactions are organic reactions in which an electron-rich substance called a nucleophile reacts with an organic substrate that has an electron-poor carbon atom called an electrophile.
The electrophile has a radical that is a weak base and is substituted by the nucleophile called the leaving group, hence the term nucleophilic substitution.
Nucleophilic substitution can occur via two different mechanisms: SN1, in which the rate determining step is the loss of the leaving group, and SN2, in which the rate-determining step is the nucleophilic attack and the simultaneous loss of the leaving group.
A scientist is studying the nucleophilic substitution reaction between 2-Bromopropane and a novel nucleophile (Nuc), collecting the kinetic data that is shown in the table below:
 Q. Using the attachment, what is the rate constant for the nucleophilic substitution reaction of 2-Bromopropane with the novel nucleophile Nuc?
Q. Using the attachment, what is the rate constant for the nucleophilic substitution reaction of 2-Bromopropane with the novel nucleophile Nuc?- a)6.42s−1
- b)0.36 s−1
- c)0.36M−1s−1
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
Nucleophilic substitution reactions are organic reactions in which an electron-rich substance called a nucleophile reacts with an organic substrate that has an electron-poor carbon atom called an electrophile.
The electrophile has a radical that is a weak base and is substituted by the nucleophile called the leaving group, hence the term nucleophilic substitution.
Nucleophilic substitution can occur via two different mechanisms: SN1, in which the rate determining step is the loss of the leaving group, and SN2, in which the rate-determining step is the nucleophilic attack and the simultaneous loss of the leaving group.
A scientist is studying the nucleophilic substitution reaction between 2-Bromopropane and a novel nucleophile (Nuc), collecting the kinetic data that is shown in the table below:

The electrophile has a radical that is a weak base and is substituted by the nucleophile called the leaving group, hence the term nucleophilic substitution.
Nucleophilic substitution can occur via two different mechanisms: SN1, in which the rate determining step is the loss of the leaving group, and SN2, in which the rate-determining step is the nucleophilic attack and the simultaneous loss of the leaving group.
A scientist is studying the nucleophilic substitution reaction between 2-Bromopropane and a novel nucleophile (Nuc), collecting the kinetic data that is shown in the table below:

Q. Using the attachment, what is the rate constant for the nucleophilic substitution reaction of 2-Bromopropane with the novel nucleophile Nuc?
a)
6.42s−1
b)
0.36 s−1
c)
0.36M−1s−1
d)
None of these
|
|
Ayesha Joshi answered |
To find out the mechanism and the rate law of the reaction, the reaction order with respect to each reactant needs to be determined using the method of initial rates. For 2-Bromopropane, trials 1 and 2 are selected and the reaction order is found in the following way:
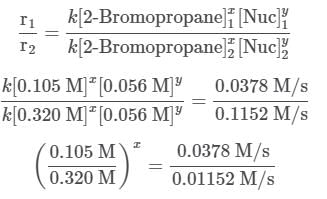
The two fractions are equivalent, hence x = 1. Now, for Nuc, trials 1 and 3 are selected, where the concentration of Nuc is kept constant, and the reaction order is found in the following way:
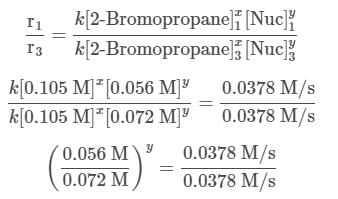
Since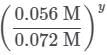 needs to be equal to 1, it means that y = 0.
needs to be equal to 1, it means that y = 0.
These reaction orders show that the reaction proceeds through an SN1 mechanism in which the rate law is r = k⋅[2 − Bromopropane].
To find out the rate constant for this reaction, any trial is selected, and the rate law is solved to find out the rate constant:

The two fractions are equivalent, hence x = 1. Now, for Nuc, trials 1 and 3 are selected, where the concentration of Nuc is kept constant, and the reaction order is found in the following way:

Since
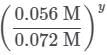 needs to be equal to 1, it means that y = 0.
needs to be equal to 1, it means that y = 0.These reaction orders show that the reaction proceeds through an SN1 mechanism in which the rate law is r = k⋅[2 − Bromopropane].
To find out the rate constant for this reaction, any trial is selected, and the rate law is solved to find out the rate constant:
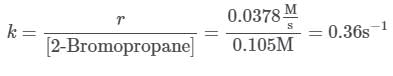
DNA (deoxyribonucleic acid) is the information-storing biomolecule of the cell. The process by which the information stored in DNA can be converted into proteins can be broken down into three subprocesses:Replication: In this process, a parent DNA strand is paired with its complementary strand. Adenine is paired with thymine, cytosine is paired with guanine, and vice-versa. In this way the information stored in DNA is preserved.
Transcription: In this process, DNA is separated and the complementary strand is copied into mRNA. Adenine is paired with uracil, cytosine is paired with guanine, and vice-versa. Translation: In this process, tRNA recognizes sequences of three bases in RNA called codons and transfers an amino acid into the growing protein chain that starts with the codon that codes for methionine. The codons are shown in the image below and are read starting from the center:
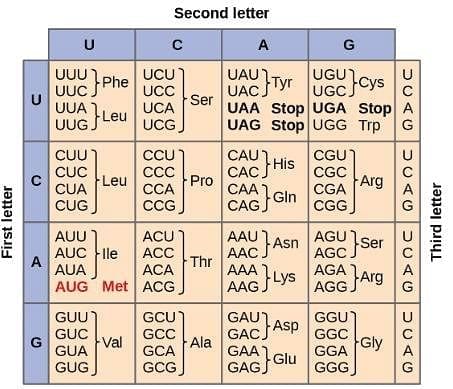 Q. Which parent DNA sequence will code for the polypeptide Met-Thr-Asp-Gly-Val? (You may consult the attachment.)
Q. Which parent DNA sequence will code for the polypeptide Met-Thr-Asp-Gly-Val? (You may consult the attachment.)- a)ATGACAGATGGAGTG
- b)TACTGTCTACCTCAC
- c)ATGACAGATTTTGTG
- d)AUGACAGAUGGAGUG
Correct answer is option 'A'. Can you explain this answer?
DNA (deoxyribonucleic acid) is the information-storing biomolecule of the cell. The process by which the information stored in DNA can be converted into proteins can be broken down into three subprocesses:
Replication: In this process, a parent DNA strand is paired with its complementary strand. Adenine is paired with thymine, cytosine is paired with guanine, and vice-versa. In this way the information stored in DNA is preserved.
Transcription: In this process, DNA is separated and the complementary strand is copied into mRNA. Adenine is paired with uracil, cytosine is paired with guanine, and vice-versa. Translation: In this process, tRNA recognizes sequences of three bases in RNA called codons and transfers an amino acid into the growing protein chain that starts with the codon that codes for methionine. The codons are shown in the image below and are read starting from the center:

Transcription: In this process, DNA is separated and the complementary strand is copied into mRNA. Adenine is paired with uracil, cytosine is paired with guanine, and vice-versa. Translation: In this process, tRNA recognizes sequences of three bases in RNA called codons and transfers an amino acid into the growing protein chain that starts with the codon that codes for methionine. The codons are shown in the image below and are read starting from the center:

Q. Which parent DNA sequence will code for the polypeptide Met-Thr-Asp-Gly-Val? (You may consult the attachment.)
a)
ATGACAGATGGAGTG
b)
TACTGTCTACCTCAC
c)
ATGACAGATTTTGTG
d)
AUGACAGAUGGAGUG
|
|
Ayesha Joshi answered |
One mRNA chain that codes for the given polypeptide is AUGACAGAUGGAGUG. The complementary DNA chain that codes for this mRNA is TACTGTCTACCTCAC, and the parent DNA strand is ATGACAGATGGAGTG.
The coordination number of the Na+ in Rock salt is- a)4
- b)3
- c)6
- d)8
- e)2
Correct answer is option 'C'. Can you explain this answer?
The coordination number of the Na+ in Rock salt is
a)
4
b)
3
c)
6
d)
8
e)
2
|
|
Ayesha Joshi answered |
In rock salt, which is the common name for sodium chloride (NaCl), the coordination number of the sodium ion (Na+) is indeed six. This means that each sodium ion is surrounded by six chloride ions (Cl-) in its immediate vicinity. Similarly, each chloride ion is surrounded by six sodium ions. This arrangement is known as a face-centered cubic (FCC) structure, where the ions are arranged in a regular and repeating pattern.
Pyruvate generated from glycolysis can follow different catabolic paths depending on many factors. In animal tissues and under anaerobic conditions, pyruvate is reduced to lactate and NADH is oxidized to NAD+, in a reaction catalyzed by lactate dehydrogenase. During the recovery period after strenuous exercise, lactate is converted back to glucose in the liver.
In an experiment, the lactate levels in blood for two athletes are measured at different times from the start of vigorous exercise. The results of the experiment are shown in the table below:
 Q. From the results of the experiment (see attachment), which athlete is in the best physical shape?
Q. From the results of the experiment (see attachment), which athlete is in the best physical shape?- a)Athlete A
- b)Both athletes are in the same physical shape.
- c)There is no way to assess physical shape from blood lactate concentrations only.
- d)Athlete B
Correct answer is option 'A'. Can you explain this answer?
Pyruvate generated from glycolysis can follow different catabolic paths depending on many factors. In animal tissues and under anaerobic conditions, pyruvate is reduced to lactate and NADH is oxidized to NAD+, in a reaction catalyzed by lactate dehydrogenase. During the recovery period after strenuous exercise, lactate is converted back to glucose in the liver.
In an experiment, the lactate levels in blood for two athletes are measured at different times from the start of vigorous exercise. The results of the experiment are shown in the table below:

In an experiment, the lactate levels in blood for two athletes are measured at different times from the start of vigorous exercise. The results of the experiment are shown in the table below:

Q. From the results of the experiment (see attachment), which athlete is in the best physical shape?
a)
Athlete A
b)
Both athletes are in the same physical shape.
c)
There is no way to assess physical shape from blood lactate concentrations only.
d)
Athlete B
|
|
Ayesha Joshi answered |
The lactate blood concentration of athlete A continues to rise after the lactate blood concentration of athlete B starts to decrease. This means that athlete A has a higher ability to provide his muscles with oxygen for a longer time, hence he is in the best physical shape.
A cell membrane defines the limits of the cell and serves as the boundary between the cell and its surroundings. It is made of a double layer of lipids that blocks the passage of polar substances. The image below shows a typical cell membrane:
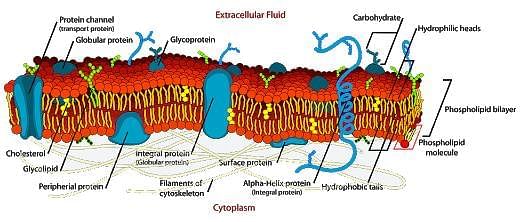 One important concept related to cell membranes is membrane fluidity. Cells need to keep a certain level of flexibility in order to maintain their stability. Increasing temperature, cholesterol content, and unsaturation of the fatty acid tail of the phospholipids in the bilayer increases the membrane fluidity.Substances pass through the cell membrane by a process called “transport”. Transport can be passive if no energy is consumed or active if energy is required to transport substances across the membrane. The movement of an uncharged solute across a membrane depends on its concentration gradient, while the movement of an ion depends both on its concentration gradient and the electric potential of the membrane. Membranes are said to be polarized if the resting membrane potential is different than zero, depolarized if the membrane potential is higher than the resting membrane potential, and hyperpolarized if the membrane potential is lower than the resting membrane potential.The following table shows the characteristics of the different types of solute transport across a membrane:
One important concept related to cell membranes is membrane fluidity. Cells need to keep a certain level of flexibility in order to maintain their stability. Increasing temperature, cholesterol content, and unsaturation of the fatty acid tail of the phospholipids in the bilayer increases the membrane fluidity.Substances pass through the cell membrane by a process called “transport”. Transport can be passive if no energy is consumed or active if energy is required to transport substances across the membrane. The movement of an uncharged solute across a membrane depends on its concentration gradient, while the movement of an ion depends both on its concentration gradient and the electric potential of the membrane. Membranes are said to be polarized if the resting membrane potential is different than zero, depolarized if the membrane potential is higher than the resting membrane potential, and hyperpolarized if the membrane potential is lower than the resting membrane potential.The following table shows the characteristics of the different types of solute transport across a membrane:
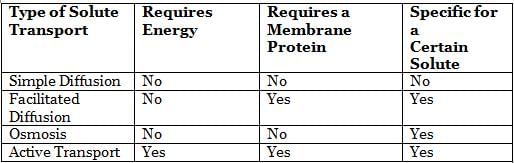 Q. When red blood cells are placed in a solution with a lower concentration of sodium ions, water molecules pass through the cell membrane from the outside to the inside of the cell in order to balance out the concentration of sodium ions. This is an example of what type of transport? (You may consult the attachment.)
Q. When red blood cells are placed in a solution with a lower concentration of sodium ions, water molecules pass through the cell membrane from the outside to the inside of the cell in order to balance out the concentration of sodium ions. This is an example of what type of transport? (You may consult the attachment.)- a)osmosis
- b)simple diffusion
- c)facilitated diffusion
- d)active transport
Correct answer is option 'A'. Can you explain this answer?
A cell membrane defines the limits of the cell and serves as the boundary between the cell and its surroundings. It is made of a double layer of lipids that blocks the passage of polar substances. The image below shows a typical cell membrane:


One important concept related to cell membranes is membrane fluidity. Cells need to keep a certain level of flexibility in order to maintain their stability. Increasing temperature, cholesterol content, and unsaturation of the fatty acid tail of the phospholipids in the bilayer increases the membrane fluidity.
Substances pass through the cell membrane by a process called “transport”. Transport can be passive if no energy is consumed or active if energy is required to transport substances across the membrane. The movement of an uncharged solute across a membrane depends on its concentration gradient, while the movement of an ion depends both on its concentration gradient and the electric potential of the membrane. Membranes are said to be polarized if the resting membrane potential is different than zero, depolarized if the membrane potential is higher than the resting membrane potential, and hyperpolarized if the membrane potential is lower than the resting membrane potential.
The following table shows the characteristics of the different types of solute transport across a membrane:


Q. When red blood cells are placed in a solution with a lower concentration of sodium ions, water molecules pass through the cell membrane from the outside to the inside of the cell in order to balance out the concentration of sodium ions. This is an example of what type of transport? (You may consult the attachment.)
a)
osmosis
b)
simple diffusion
c)
facilitated diffusion
d)
active transport
|
|
Ayesha Joshi answered |
Since the concentration of sodium ions is being balanced out, this form of transport does not require energy. The transport is specific for water molecules and they are passing through the cell membrane without the help of a membrane protein, so this form of transport is classified as osmosis.
The Henderson-Hasselbalch equation for acid is- a)pH = pKa- log ([A-] / [HA])
- b)pH = pKa + log ([HA] / [A-])
- c)pH = pKb + log ([B] / [HB+])
- d)pH = pKa + log ([A-] / [HA])
- e)pH = pKa + 2log ([A-] / [HA])
Correct answer is option 'D'. Can you explain this answer?
The Henderson-Hasselbalch equation for acid is
a)
pH = pKa- log ([A-] / [HA])
b)
pH = pKa + log ([HA] / [A-])
c)
pH = pKb + log ([B] / [HB+])
d)
pH = pKa + log ([A-] / [HA])
e)
pH = pKa + 2log ([A-] / [HA])
|
|
Ayesha Joshi answered |
The Henderson-Hasselbalch equation explains whether a compound will exist in its acidic form or in its basic form at a particular pH. [A-] = Molar concentration of a conjugate base. [HA] = Molar concentration of an undissociated weak acid (M).
What is the oxidation number of sulfur in the compound H2SO4?- a)-1
- b)+2
- c)+4
- d)+6
Correct answer is option 'D'. Can you explain this answer?
What is the oxidation number of sulfur in the compound H2SO4?
a)
-1
b)
+2
c)
+4
d)
+6
|
|
Ayesha Joshi answered |
In H2SO4 (sulfuric acid), the hydrogen atoms have an oxidation number of +1, and the oxygen atoms have an oxidation number of -2. To find the oxidation number of sulfur, we can set up the equation:
(+1) * 2 + x + (-2) * 4 = 0
Simplifying the equation gives:
2 + x - 8 = 0
x - 6 = 0
x = 6
Therefore, the oxidation number of sulfur in H2SO4 is +6.
(+1) * 2 + x + (-2) * 4 = 0
Simplifying the equation gives:
2 + x - 8 = 0
x - 6 = 0
x = 6
Therefore, the oxidation number of sulfur in H2SO4 is +6.
Which of the following lipids is not found in the cell membrane?- a)phospholipids
- b)sphingolipids
- c)cholesterol
- d)triacylglycerols
Correct answer is option 'D'. Can you explain this answer?
Which of the following lipids is not found in the cell membrane?
a)
phospholipids
b)
sphingolipids
c)
cholesterol
d)
triacylglycerols
|
|
Ayesha Joshi answered |
Cholesterol, phospholipids, and sphingolipids have a polar head and a nonpolar tail and are part of the cell membrane’s bilayer. Triacylglycerols have a nonpolar tail made of fatty acids linked to glycerol, but they lack a polar head so they are not found in the cell membrane.
Hess's law is related to ________ of the system.- a)Free energy change
- b)Entropy change
- c)Enthalpy change
- d)Internal energy
- e)None of these
Correct answer is option 'C'. Can you explain this answer?
Hess's law is related to ________ of the system.
a)
Free energy change
b)
Entropy change
c)
Enthalpy change
d)
Internal energy
e)
None of these
|
|
Ayesha Joshi answered |
Hess's law states that the enthalpy or heat energy change accompanying a chemical reaction is independent of the pathway between the initial and final states. ΔH for a single reaction can be calculated from the difference between the enthalpy (heat) of formation of the product and reactant.
ΔH0 reaction = Σ ΔHf0 (products) – Σ ΔHf0 (reactant)
ΔH0 reaction = Σ ΔHf0 (products) – Σ ΔHf0 (reactant)
Which of the following statements correctly describes an exothermic reaction?- a)It absorbs heat from the surroundings.
- b)It releases heat to the surroundings.
- c)It neither absorbs nor releases heat.
- d)It involves the transfer of light energy.
Correct answer is option 'B'. Can you explain this answer?
Which of the following statements correctly describes an exothermic reaction?
a)
It absorbs heat from the surroundings.
b)
It releases heat to the surroundings.
c)
It neither absorbs nor releases heat.
d)
It involves the transfer of light energy.
|
|
Ayesha Joshi answered |
In an exothermic reaction, energy is released in the form of heat to the surroundings. The reactants have a higher energy level compared to the products, and the excess energy is given off as heat. This is why exothermic reactions often feel warm or hot to the touch.
Among the following, which group of elements have the maximum ionisation energy?- a)Alkali elements
- b)Chalcogens
- c)Halogens
- d)Noble gases
- e)Both b and c
Correct answer is option 'D'. Can you explain this answer?
Among the following, which group of elements have the maximum ionisation energy?
a)
Alkali elements
b)
Chalcogens
c)
Halogens
d)
Noble gases
e)
Both b and c
|
|
Ayesha Joshi answered |
Ionization energy refers to the energy needed to remove the least tightly bound electron from an isolated atom in its gaseous state. In a periodic table period, the ionization potential generally increases from left to right. This means that as you move from left to right across a period, it becomes more difficult to remove an electron from the atoms. Noble gases, which are located on the far right of the periodic table, tend to have the highest ionization energies compared to other elements because their electron configuration is highly stable with completely filled valence shells.
Chapter doubts & questions for Chemistry - MCAT Mock Test Series 2025 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemistry - MCAT Mock Test Series 2025 in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily









