All Exams >
JEE >
Online MCQ Tests for JEE >
All Questions
All questions of Nuclei for JEE Exam
Nucleus ”a” contains 5 protons and 5 neutrons and has radius R. The radius of nucleus ”b”, which contains 35 protons and 45 neutrons, is closest to:- a)2R
- b)8R
- c)1.4R
- d)R
Correct answer is option 'A'. Can you explain this answer?
Nucleus ”a” contains 5 protons and 5 neutrons and has radius R. The radius of nucleus ”b”, which contains 35 protons and 45 neutrons, is closest to:
a)
2R
b)
8R
c)
1.4R
d)
R
|
|
Gaurav Kumar answered |
R∝A1/3
A(mass no.)=n+p
R/x=(10/80)1/3
R/x=(13/23)1/3
R/x=1/2
X=2R
A(mass no.)=n+p
R/x=(10/80)1/3
R/x=(13/23)1/3
R/x=1/2
X=2R
A sample of radioactive material contains 1018 atoms. The half life of the material is 2 days, then the activity of the sample is- a)3.5 x 1014 Bq
- b)3.5 x 1012 Bq
- c)7 x 1011 Bq
- d)7 x 1016 Bq
Correct answer is option 'B'. Can you explain this answer?
A sample of radioactive material contains 1018 atoms. The half life of the material is 2 days, then the activity of the sample is
a)
3.5 x 1014 Bq
b)
3.5 x 1012 Bq
c)
7 x 1011 Bq
d)
7 x 1016 Bq
|
|
Jyoti Sengupta answered |
To find activity of the sample --->which is the rate of disintegration.
Since radioactivity comes under 1o kinetics.
[R]=k[A] [A]-->amount of initial sample 1018 atoms
Given,
Half-life=2days
K=0.693/2x24x60x60 sec
R=(0.693/2x24x60x60)x1018
R≈3.5x1012 Bq
A radioactive material decays by simultaneous emission of two particles with respective half lives 1620 and 810 years. The time in years, after which one fourth of the material remains is- a)4860
- b)2340
- c)1080.0
- d)3240
Correct answer is option 'C'. Can you explain this answer?
A radioactive material decays by simultaneous emission of two particles with respective half lives 1620 and 810 years. The time in years, after which one fourth of the material remains is
a)
4860
b)
2340
c)
1080.0
d)
3240
|
|
Nikita Singh answered |
Since, from Rutherford-Soddy law, the number of atoms left after half-lives is given by
N=N0(1/2)n
where, N0 is the original number of atoms.
The number of half-lives, n= time of decay/effective half−life
Relation between effective disintegration constant (λ) and half-life (T)
λ=ln2/T
∴λ1+λ2= (ln2/ T1)+ (ln2/ T2)
Effective half-life,
1/T=1/T1+1/T2=(1/1620)+(1/810)
1/T=1+2/1620 ⇒T=540yr
∴n=T/540
∴N=N0(1/2)t/540⇒N/N0=(1/2)2=(1/2)t/540
⇒t/540=2⇒t=2×540=1080yr
N=N0(1/2)n
where, N0 is the original number of atoms.
The number of half-lives, n= time of decay/effective half−life
Relation between effective disintegration constant (λ) and half-life (T)
λ=ln2/T
∴λ1+λ2= (ln2/ T1)+ (ln2/ T2)
Effective half-life,
1/T=1/T1+1/T2=(1/1620)+(1/810)
1/T=1+2/1620 ⇒T=540yr
∴n=T/540
∴N=N0(1/2)t/540⇒N/N0=(1/2)2=(1/2)t/540
⇒t/540=2⇒t=2×540=1080yr
The number of electrons in an atom X of atomic number Z and mass number A is- a)Zero
- b)A
- c)Z
- d)A-Z
Correct answer is option 'C'. Can you explain this answer?
The number of electrons in an atom X of atomic number Z and mass number A is
a)
Zero
b)
A
c)
Z
d)
A-Z

|
Sushil Kumar answered |
No of neutrons are given by: (A−Z)
Given an atomic number (Z) and mass number (A), you can find the number of protons, neutrons, and electrons in a neutral atom. For example, a lithium atom (Z=3,A=7 amu) contains three protons (found from Z), three electrons (as the number of protons is equal to the number of electrons in an atom), and four neutrons (7–3=4).
Given an atomic number (Z) and mass number (A), you can find the number of protons, neutrons, and electrons in a neutral atom. For example, a lithium atom (Z=3,A=7 amu) contains three protons (found from Z), three electrons (as the number of protons is equal to the number of electrons in an atom), and four neutrons (7–3=4).
Cadmium rods are used in a nuclear reactor for- a)absorbing neutrons
- b)speeding up slow neutrons
- c)regulating the power level of the reactor.
- d)slowing down fast neutrons
Correct answer is option 'A'. Can you explain this answer?
Cadmium rods are used in a nuclear reactor for
a)
absorbing neutrons
b)
speeding up slow neutrons
c)
regulating the power level of the reactor.
d)
slowing down fast neutrons

|
Awantika Gupta answered |
Cadmium and boron rod both are used in cotroling the reactivity of uranium means slow down the rate of fission...
All nuclides with same mass number A are called- a)isobars
- b)isoclines
- c)isotones
- d)isotopes
Correct answer is option 'A'. Can you explain this answer?
All nuclides with same mass number A are called
a)
isobars
b)
isoclines
c)
isotones
d)
isotopes
|
|
Rocky Handsome answered |
Isobars are atoms of different elements with the same mass number but different atomic numbers.
• Isotones are atomic nuclei with the same number of neutrons (N) and different number of protons(Z)
• Isotones are atomic nuclei with the same number of neutrons (N) and different number of protons(Z)
90% of a radioactive sample is left undisintegrated after time τ has elapsed, what percentage of initial sample will decay in a total time2τ?- a)9%
- b)38%
- c)19%
- d)62%
Correct answer is option 'C'. Can you explain this answer?
90% of a radioactive sample is left undisintegrated after time τ has elapsed, what percentage of initial sample will decay in a total time2τ?
a)
9%
b)
38%
c)
19%
d)
62%
|
|
Krishna Iyer answered |
Given that 90% is left un-decayed after time 't'.
Hence, 10% decays in time 't'.
Initially assume that the amount of substance is 'x'
After time 't' 10% is decayed.
i.e. Amount of substance left =0.9x
After further time 't' another 10% is decayed.
i.e. 0.1×0.9x is decayed
Leaving behind 0.81x.
Hence after time 2t we see that 0.19x has decayed, which is 19%.
Hence, 10% decays in time 't'.
Initially assume that the amount of substance is 'x'
After time 't' 10% is decayed.
i.e. Amount of substance left =0.9x
After further time 't' another 10% is decayed.
i.e. 0.1×0.9x is decayed
Leaving behind 0.81x.
Hence after time 2t we see that 0.19x has decayed, which is 19%.
The nuclide 92U238 has all the following except- a)92 protons
- b)146 neutrons
- c)238 nucleons
- d)92 neutrons
Correct answer is option 'D'. Can you explain this answer?
The nuclide 92U238 has all the following except
a)
92 protons
b)
146 neutrons
c)
238 nucleons
d)
92 neutrons

|
Sushil Kumar answered |
The nuclide (nucleus) consists of neutrons and protons (when combined called nucleons).
Thus,
No. of protons in 92U238 = 92,
No. of neutrons = 146 (238 – 92)
No. of nucleons = 238 (146 + 92)
Thus,
No. of protons in 92U238 = 92,
No. of neutrons = 146 (238 – 92)
No. of nucleons = 238 (146 + 92)
α-rays are- a)helium nuclei
- b)heavy nuclei
- c)lithium nuclei
- d)hydrogen nuclei
Correct answer is option 'A'. Can you explain this answer?
α-rays are
a)
helium nuclei
b)
heavy nuclei
c)
lithium nuclei
d)
hydrogen nuclei
|
|
Ræjû Bhæï answered |
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways.
At a given time there are 25% undecayed nuclei in a sample. After 10 seconds number of undecayed nuclei reduces to 12.5%. Then mean life of the nuclei will be about- a)22 sec
- b)10 sec
- c)12 sec
- d)15 sec
Correct answer is option 'D'. Can you explain this answer?
At a given time there are 25% undecayed nuclei in a sample. After 10 seconds number of undecayed nuclei reduces to 12.5%. Then mean life of the nuclei will be about
a)
22 sec
b)
10 sec
c)
12 sec
d)
15 sec
|
|
Lavanya Menon answered |
Half-life of radioactive sample, i.e., the time in which the number of undecayed nuclei becomes half (T) is 10 s.
Mean life, τ=T/loge2=10s/0.693=1.443×10=14.43s ≈ 15s
Mean life, τ=T/loge2=10s/0.693=1.443×10=14.43s ≈ 15s
The half life of radon is 3.8 days. After how many days will only one twentieth of a radon sample be left over?
a)10.00 daysb)5.45 daysc)15.45 daysd)16.45 daysCorrect answer is option 'D'. Can you explain this answer?
|
|
Suresh Iyer answered |
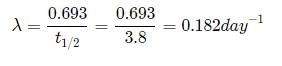 Let the initial amount of radon be N0 and the amount left after t days be N which is equal to N0/2
Let the initial amount of radon be N0 and the amount left after t days be N which is equal to N0/2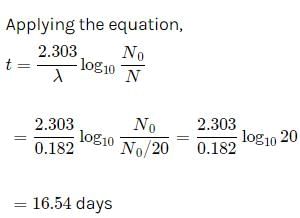
In the following reaction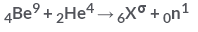 What is following value of a?
What is following value of a?- a)14
- b)10
- c)16
- d)12
Correct answer is option 'D'. Can you explain this answer?
In the following reaction
What is following value of a?
a)
14
b)
10
c)
16
d)
12
|
|
Tanuja Kapoor answered |
The sum of the atomic no. and atomic mass no. on the reactant and product should be equal .
therefore 9+4 = a+1
a = 12
therefore 9+4 = a+1
a = 12
The atomic number Z of the nucleus is- a)Number of deutrons.
- b)Number of neutrons in it.
- c)Number if electrons in it.
- d)Number of protons in it.
Correct answer is option 'D'. Can you explain this answer?
The atomic number Z of the nucleus is
a)
Number of deutrons.
b)
Number of neutrons in it.
c)
Number if electrons in it.
d)
Number of protons in it.
|
|
Shraddha Dey answered |
**Explanation:**
The atomic number (Z) of an atom refers to the number of protons in the nucleus. Here, we will discuss why the correct answer is option 'D' and explain the significance of atomic number in an atom.
**Atomic Number (Z):**
The atomic number of an atom is a fundamental property that determines its identity and place in the periodic table. It is denoted by the symbol 'Z'. Each element on the periodic table has a unique atomic number.
**Protons in the Nucleus:**
Protons are subatomic particles that carry a positive charge. They are located in the nucleus of an atom, which is the central core of the atom. The number of protons in the nucleus is equal to the atomic number of the atom.
**Electrons in the Atom:**
Electrons are subatomic particles that carry a negative charge. They orbit around the nucleus in specific energy levels or shells. The number of electrons in a neutral atom is equal to the number of protons, ensuring that the atom has a balanced charge overall.
**Neutrons in the Nucleus:**
Neutrons are subatomic particles that have no charge (they are electrically neutral). They are also located in the nucleus along with protons. The number of neutrons in an atom can vary, resulting in different isotopes of the same element. Isotopes have the same atomic number (same number of protons) but different mass numbers (different number of neutrons).
**Significance of Atomic Number:**
The atomic number is a crucial characteristic of an atom because it determines the element's identity. Elements are organized in increasing order of their atomic numbers on the periodic table. For example, hydrogen has an atomic number of 1, helium has an atomic number of 2, and so on.
The atomic number defines the unique properties and behavior of an element. It determines the number of electrons in the atom, which influences the atom's chemical reactivity and bonding. It also provides information about the element's position in the periodic table, its atomic mass, and its isotopes.
Therefore, the correct answer to the given question is option 'D' – the atomic number (Z) of the nucleus represents the number of protons in it.
The atomic number (Z) of an atom refers to the number of protons in the nucleus. Here, we will discuss why the correct answer is option 'D' and explain the significance of atomic number in an atom.
**Atomic Number (Z):**
The atomic number of an atom is a fundamental property that determines its identity and place in the periodic table. It is denoted by the symbol 'Z'. Each element on the periodic table has a unique atomic number.
**Protons in the Nucleus:**
Protons are subatomic particles that carry a positive charge. They are located in the nucleus of an atom, which is the central core of the atom. The number of protons in the nucleus is equal to the atomic number of the atom.
**Electrons in the Atom:**
Electrons are subatomic particles that carry a negative charge. They orbit around the nucleus in specific energy levels or shells. The number of electrons in a neutral atom is equal to the number of protons, ensuring that the atom has a balanced charge overall.
**Neutrons in the Nucleus:**
Neutrons are subatomic particles that have no charge (they are electrically neutral). They are also located in the nucleus along with protons. The number of neutrons in an atom can vary, resulting in different isotopes of the same element. Isotopes have the same atomic number (same number of protons) but different mass numbers (different number of neutrons).
**Significance of Atomic Number:**
The atomic number is a crucial characteristic of an atom because it determines the element's identity. Elements are organized in increasing order of their atomic numbers on the periodic table. For example, hydrogen has an atomic number of 1, helium has an atomic number of 2, and so on.
The atomic number defines the unique properties and behavior of an element. It determines the number of electrons in the atom, which influences the atom's chemical reactivity and bonding. It also provides information about the element's position in the periodic table, its atomic mass, and its isotopes.
Therefore, the correct answer to the given question is option 'D' – the atomic number (Z) of the nucleus represents the number of protons in it.
The radius of a nucleus is directly proportional to (A=mass number)- a)A1/3
- b)A2
- c)A3
- d)A1/2
Correct answer is option 'A'. Can you explain this answer?
The radius of a nucleus is directly proportional to (A=mass number)
a)
A1/3
b)
A2
c)
A3
d)
A1/2
|
|
Riya Banerjee answered |
For A nucleons
R=RoA1/3 [Ro=constant]
So, R∝A1/3
R=RoA1/3 [Ro=constant]
So, R∝A1/3
What is the main source of energy of the sun?- a)Nuclear fission of heavier unstable elements in the sun
- b)Combustion of pure carbon present in the sun
- c)Gravitational energy liberated during the slow contraction of the sun.
- d)Nuclear fusion of lighter elements in the sun.
Correct answer is option 'D'. Can you explain this answer?
What is the main source of energy of the sun?
a)
Nuclear fission of heavier unstable elements in the sun
b)
Combustion of pure carbon present in the sun
c)
Gravitational energy liberated during the slow contraction of the sun.
d)
Nuclear fusion of lighter elements in the sun.

|
Kanika S answered |
Option d
When H and He in sun undergoes fusion large amount of heat is released.
When H and He in sun undergoes fusion large amount of heat is released.
The average number of neutrons released by the fission of one uranium atom is
a)3.0b)2c)2.5d)1Correct answer is option 'C'. Can you explain this answer?
|
|
Bhanu Saini answered |
Fission result in the production of typically 2 or 3 neutron so on the average about 2.5 neutron released per unit. so correct answer is option a
for option c one uranium atom split into one barium and one krypton atom releasing 3 neutron.
but in this question average is asking so according to me and books 2.5 is correct
for option c one uranium atom split into one barium and one krypton atom releasing 3 neutron.
but in this question average is asking so according to me and books 2.5 is correct
Nuclear mass M is found to be- a)always greater than total mass of its individual protons and neutrons
- b)always equal to the total mass of its individual neutrons
- c)always equal to the total mass of its individual protons and neutrons
- d)always less than total mass of its individual protons and neutrons
Correct answer is option 'D'. Can you explain this answer?
Nuclear mass M is found to be
a)
always greater than total mass of its individual protons and neutrons
b)
always equal to the total mass of its individual neutrons
c)
always equal to the total mass of its individual protons and neutrons
d)
always less than total mass of its individual protons and neutrons
|
|
Ritu Singh answered |
The actual mass is always less than the sum of the individual masses of the constituent protons and neutrons because energy is removed when the nucleus is formed. This energy has mass, which is removed from the total mass of the original particles.
What percentage of the mass of an atom is concentrated in the nucleus?- a)79.9%
- b)99.9%
- c)66.9%
- d)50.9%
Correct answer is option 'B'. Can you explain this answer?
What percentage of the mass of an atom is concentrated in the nucleus?
a)
79.9%
b)
99.9%
c)
66.9%
d)
50.9%
|
|
Jyoti Kapoor answered |
More than 99.99% of the mass of any atom is concentrated in its nucleus. If the mass of protons and neutrons (which are in the nucleus of every atom) is approximately one (1) atomic mass unit, then the relative mass of an electron is 0.0005 atomic mass units.
What amount of energy is released in the fission of 95U235 ?- a)200 keV
- b)20 eV
- c)200 eV
- d)200 MeV
Correct answer is option 'D'. Can you explain this answer?
What amount of energy is released in the fission of 95U235 ?
a)
200 keV
b)
20 eV
c)
200 eV
d)
200 MeV

|
Divey Sethi answered |
The fission process represented by the equation, 92U235+0n1→56Ba144+36Kr89+30n1
Masses of reactants =234.39+1.01=235.4amu
Masses of products =143.28+88.89+3(1.01) =235.2amu
Energy released = mass difference =235.4−235.2=0.2amu=0.2×931∼200MeV
Masses of reactants =234.39+1.01=235.4amu
Masses of products =143.28+88.89+3(1.01) =235.2amu
Energy released = mass difference =235.4−235.2=0.2amu=0.2×931∼200MeV
Plutonium decays with a half-life of 24000 years. If the plutonium is stored for 72000 years, then the fraction of plutonium that remains is - a)1 /3
- b)1 /2
- c)1/8
- d)1 /4
Correct answer is option 'C'. Can you explain this answer?
Plutonium decays with a half-life of 24000 years. If the plutonium is stored for 72000 years, then the fraction of plutonium that remains is
a)
1 /3
b)
1 /2
c)
1/8
d)
1 /4
|
|
Mira Sharma answered |
The amount of plotinium after a time period of 72000 if the half life is 24000 will be
the initial amount x would be reduced to x/2 , in 24000 yrs
then it would lessen to x/4 in the next 24000yrs
and then to x/8 in the next 24000 yrs
that is it will reduce to x/8 in the next 72000yrs starting from x .
In the mass number range A = 30 to 170, the binding energy per nucleon is- a)decreases with increasing A
- b)increases linearly with A
- c)decreases linearly with A
- d)nearly constant
Correct answer is option 'D'. Can you explain this answer?
In the mass number range A = 30 to 170, the binding energy per nucleon is
a)
decreases with increasing A
b)
increases linearly with A
c)
decreases linearly with A
d)
nearly constant
|
|
Shraddha Choudhury answered |
Binding energy per nucleon in the mass number range A = 30 to 170
The binding energy per nucleon is the energy required to separate a nucleus into its constituent nucleons. It is a measure of the stability of the nucleus, and it depends on the mass number of the nucleus. In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
Explanation:
The binding energy per nucleon is given by the formula:
BE/A = (ZmH + NmN - M)/A
where BE is the binding energy, Z is the atomic number, N is the number of neutrons, mH is the mass of a hydrogen atom, mN is the mass of a neutron, and M is the mass of the nucleus.
In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant because the nuclear force between nucleons is nearly constant. This means that the energy required to separate a nucleon from the nucleus is nearly constant in this range.
The nuclear force between nucleons is a strong force that holds the nucleus together. It is a short-range force that depends on the distance between nucleons. In the mass number range A = 30 to 170, the distance between nucleons is nearly constant, and so the nuclear force is nearly constant.
Therefore, the binding energy per nucleon is nearly constant in this range because the nuclear force is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
The binding energy per nucleon is the energy required to separate a nucleus into its constituent nucleons. It is a measure of the stability of the nucleus, and it depends on the mass number of the nucleus. In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
Explanation:
The binding energy per nucleon is given by the formula:
BE/A = (ZmH + NmN - M)/A
where BE is the binding energy, Z is the atomic number, N is the number of neutrons, mH is the mass of a hydrogen atom, mN is the mass of a neutron, and M is the mass of the nucleus.
In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant because the nuclear force between nucleons is nearly constant. This means that the energy required to separate a nucleon from the nucleus is nearly constant in this range.
The nuclear force between nucleons is a strong force that holds the nucleus together. It is a short-range force that depends on the distance between nucleons. In the mass number range A = 30 to 170, the distance between nucleons is nearly constant, and so the nuclear force is nearly constant.
Therefore, the binding energy per nucleon is nearly constant in this range because the nuclear force is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
In what units is mass measured on the atomic scale?- a)kilogram
- b)atomic mass units (u)
- c)milligram
- d)gram
Correct answer is option 'B'. Can you explain this answer?
In what units is mass measured on the atomic scale?
a)
kilogram
b)
atomic mass units (u)
c)
milligram
d)
gram
|
|
Lavanya Menon answered |
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.
The nuclei of isotopes of a given element contain the same number of- a)neutrinos
- b)protons
- c)neutrons
- d)positrons
Correct answer is option 'B'. Can you explain this answer?
The nuclei of isotopes of a given element contain the same number of
a)
neutrinos
b)
protons
c)
neutrons
d)
positrons
|
|
Varanasi Sai Srinivasa K answered |
Atom of same element, contain same number of protons, they differ in number of neutrons .
This is known as isotope .
Therefore we can conclude that answer is [ B ]
This is known as isotope .
Therefore we can conclude that answer is [ B ]
The average binding energy of nucleus is- a)8 BeV
- b)8 Mev
- c)8 eV
- d)8 KeV
Correct answer is option 'B'. Can you explain this answer?
The average binding energy of nucleus is
a)
8 BeV
b)
8 Mev
c)
8 eV
d)
8 KeV
|
|
Rahul Bansal answered |
Excluding the lighter nuclei, the average binding energy per nucleon is about 8 MeV. The maximum binding energy per nucleon occurs at around mass number A = 50, and corresponds to the most stable nuclei.
γ (Gamma) rays are- a)Singly ionized gas atoms
- b)Electromagnetic waves
- c)Helium nuclei
- d)Fast moving electrons
Correct answer is option 'B'. Can you explain this answer?
γ (Gamma) rays are
a)
Singly ionized gas atoms
b)
Electromagnetic waves
c)
Helium nuclei
d)
Fast moving electrons
|
|
Prakhar Inani answered |
Since gamma rays carry energy that's why they are electromagnetic waves.
The chemical behavior of an atom depends upon- a)the number of neutrons in its nucleus
- b)the number of nucleons in its nucleus.
- c)the number of electrons orbiting around its nucleus
- d)the number of protons in its nucleus
Correct answer is option 'C'. Can you explain this answer?
The chemical behavior of an atom depends upon
a)
the number of neutrons in its nucleus
b)
the number of nucleons in its nucleus.
c)
the number of electrons orbiting around its nucleus
d)
the number of protons in its nucleus

|
Kuheli Sengupta answered |
This is because in most chemical reactions only electrons participate. the nucleons have little role in chemical reactions
Given M = mass of the nucleus, A = atomic mass. What is packing fraction?- a)
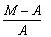
- b)
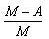
- c)
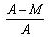
- d)
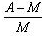
Correct answer is option 'A'. Can you explain this answer?
Given M = mass of the nucleus, A = atomic mass. What is packing fraction?
a)
b)
c)
d)

|
Infinity Academy answered |
Packing fraction: - It tells about the stability of a nucleus.
Packing fraction=isotonic mass=molecular mass (atomic mass)/atomic mass
p.f.=M-A/A
Packing fraction=isotonic mass=molecular mass (atomic mass)/atomic mass
p.f.=M-A/A
Which of the following statements is true for nuclear forces?- a)They are short range forces
- b)They are equal in strength to the electromagnetic forces
- c)They obey the inverse third power law of distance
- d)They obey the inverse square law of distance
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements is true for nuclear forces?
a)
They are short range forces
b)
They are equal in strength to the electromagnetic forces
c)
They obey the inverse third power law of distance
d)
They obey the inverse square law of distance
|
|
Om Desai answered |
Nuclear forces are short range forces. This is the only correct answer. Others are wrong. They are the strongest forces in nature and do not obey inverse square law.
Nuclear fusion is possible- a)only between light nuclei
- b)only between heavy nuclei
- c)between both light and heavy nuclei
- d)only between nuclei which are stable against β-decay
Correct answer is option 'A'. Can you explain this answer?
Nuclear fusion is possible
a)
only between light nuclei
b)
only between heavy nuclei
c)
between both light and heavy nuclei
d)
only between nuclei which are stable against β-decay
|
|
Harshit Agrawal answered |
In nuclear fusion, two or more small nuclei combine to form a single larger nucleus, a neutron, and a tremendous amount of energy. Nuclear fusion of hydrogen to form helium occurs naturally in the sun and other stars. It takes place only at extremely high temperatures.
B210has a half life of 5 days. The time taken for seven-eighth of a sample to decay is- a)10 days
- b)20 days
- c)3.4 days
- d)15 days
Correct answer is option 'D'. Can you explain this answer?
B210has a half life of 5 days. The time taken for seven-eighth of a sample to decay is
a)
10 days
b)
20 days
c)
3.4 days
d)
15 days
|
|
Srishti Chavan answered |
Half-life of Bi210=5 days
∴k= 0.693/(t1/2) =(0.693/5) day−1
Using k=(2.303/t) log (a/a-x)
(where a = a0, (let) ⇒x=7/8 a0, t is time taken in decay and k is rate constant)
We get, t=(2.303×5/0.693)log a0/(1/8)a0
= (2.303×5/0.693) log8=15days
∴k= 0.693/(t1/2) =(0.693/5) day−1
Using k=(2.303/t) log (a/a-x)
(where a = a0, (let) ⇒x=7/8 a0, t is time taken in decay and k is rate constant)
We get, t=(2.303×5/0.693)log a0/(1/8)a0
= (2.303×5/0.693) log8=15days
Which one of the following statements about the atomic nucleus is accurate?- a)The nucleus is held together mostly by the electrical and gravitational forces
- b)Large nuclei are denser than light nuclei
- c)All nuclei have nearly the same density
- d)Smaller nuclei are denser than larger nuclei
Correct answer is option 'C'. Can you explain this answer?
Which one of the following statements about the atomic nucleus is accurate?
a)
The nucleus is held together mostly by the electrical and gravitational forces
b)
Large nuclei are denser than light nuclei
c)
All nuclei have nearly the same density
d)
Smaller nuclei are denser than larger nuclei
|
|
Riya Banerjee answered |
Most nuclei are approximately spherical. The average radius of a nucleus with A nucleons is R = R0A1/3, where R0 = 1.2*10-15 m. The volume of the nucleus is directly proportional to the total number of nucleons. This suggests that all nuclei have nearly the same density.
When a radioactive nucleus emits a β – particle, the mass number of the atom- a)Increases by one
- b)Decreases by one
- c)Remain the same
- d)Decreaseds by four
Correct answer is option 'C'. Can you explain this answer?
When a radioactive nucleus emits a β – particle, the mass number of the atom
a)
Increases by one
b)
Decreases by one
c)
Remain the same
d)
Decreaseds by four
|
|
Saloni Mishra answered |
C Mass no Remains same ,there is an increase of +1 unit in atomic no
The density of nuclear matter is:- a)independent of the number of nucleons in the nucleus
- b)directly proportional to the number of neutrons in the nucleus
- c)directly proportional to the number of protons in the nucleus
- d)directly proportional to the square of the number of nucleons in the nucleus
Correct answer is option 'A'. Can you explain this answer?
The density of nuclear matter is:
a)
independent of the number of nucleons in the nucleus
b)
directly proportional to the number of neutrons in the nucleus
c)
directly proportional to the number of protons in the nucleus
d)
directly proportional to the square of the number of nucleons in the nucleus
|
|
Pooja Mehta answered |
Nuclear density is the density of the nucleus of an atom. It is the ratio of mass per unit volume inside the nucleus. Since atomic nucleus carries most of atom’s mass and atomic nucleus is very small in comparison to entire atom, the nuclear density is very high.
The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and from its mass. Typical nuclear radii are of the order 10−14 m. Assuming spherical shape, nuclear radii can be calculated according to following formula:
r = r0 . A1/3
where r0 = 1.2 x 10-15 m = 1.2 fm
For example, natural uranium consists primarily of isotope 238U (99.28%), therefore the atomic mass of uranium element is close to the atomic mass of 238U isotope (238.03u). Its radius of this nucleus will be:
r = r0 . A1/3 = 7.44 fm.
Assuming it is spherical, its volume will be:
V = 4πr3/3 = 1.73 x 10-42 m3.
The usual definition of nuclear density gives for its density:
ρnucleus = m / V = 238 x 1.66 x 10-27 / (1.73 x 10-42) = 2.3 x 1017 kg/m3.
Thus, the density of nuclear material is more than 2.1014 times greater than that of water. It is an immense density. The descriptive term nuclear density is also applied to situations where similarly high densities occur, such as within neutron stars. Such immense densities are also found in neutron stars.
Artificial disintegration with alpha particles of which of the following led to the discovery of neutron?- a)Be
- b)Na
- c)N
- d)Ba
Correct answer is option 'A'. Can you explain this answer?
Artificial disintegration with alpha particles of which of the following led to the discovery of neutron?
a)
Be
b)
Na
c)
N
d)
Ba
|
|
Anjali Sharma answered |
James Chadwick set up an experiment to test his hypothesis. Chadwick put a piece of beryllium in a vacuum chamber with some polonium. The polonium emitted alpha rays, which struck the beryllium. When struck, the beryllium emitted the mysterious neutral rays.
Choose the WRONG statement. A thermonuclear fusion reactor is better than a fission reactor for the following reason:- a)For the same mass of substances involved, a fusion reaction releases much more energy than a fission reaction.
- b)The fuel required for fusion is readily available in abundance from seawater.
- c)A fusion reaction can be much more easily controlled than a fission
- d)A fusion reaction produces almost no radioactive waste.
Correct answer is option 'B'. Can you explain this answer?
Choose the WRONG statement. A thermonuclear fusion reactor is better than a fission reactor for the following reason:
a)
For the same mass of substances involved, a fusion reaction releases much more energy than a fission reaction.
b)
The fuel required for fusion is readily available in abundance from seawater.
c)
A fusion reaction can be much more easily controlled than a fission
d)
A fusion reaction produces almost no radioactive waste.
|
|
Nisha Kulkarni answered |
Explanation:
The wrong statement among the given options is option B: "The fuel required for fusion is readily available in abundance from seawater."
Reason:
- While it is correct that a thermonuclear fusion reactor is better than a fission reactor for several reasons, including higher energy release and less radioactive waste production, the availability of fuel from seawater is not accurate.
- Fusion reactions require isotopes of hydrogen, such as deuterium and tritium, as fuel. Deuterium can be extracted from seawater, but tritium is a radioactive isotope that is not naturally abundant and needs to be produced artificially.
- Tritium can be produced by exposing lithium to neutron radiation, which can be generated by a fission reactor or a fusion reactor itself. However, the process of producing tritium is not as straightforward as extracting deuterium from seawater.
- Tritium is also highly radioactive and has a short half-life, which means it requires careful handling and containment. It cannot be easily stored or transported.
- Therefore, the fuel required for fusion reactions is not readily available in abundance from seawater, as stated in option B.
Correct statements:
a) For the same mass of substances involved, a fusion reaction releases much more energy than a fission reaction.
- This is true. Fusion reactions release a tremendous amount of energy, several times more than fission reactions. The fusion of hydrogen atoms into helium is the same process occurring in the Sun and other stars, which produces immense amounts of energy.
c) A fusion reaction can be much more easily controlled than a fission reaction.
- This is true. Fusion reactions require extremely high temperatures and pressures to sustain, and if these conditions are not maintained, the reaction will cease. This inherent stability makes fusion reactions more easily controllable than fission reactions, which can lead to runaway chain reactions if not properly regulated.
d) A fusion reaction produces almost no radioactive waste.
- This is true. Fusion reactions do not produce long-lived radioactive waste like fission reactions. The only radioactive byproduct of fusion is tritium, which has a relatively short half-life and can be managed safely.
In summary, option B is the wrong statement because the fuel required for fusion reactions is not readily available in abundance from seawater.
The wrong statement among the given options is option B: "The fuel required for fusion is readily available in abundance from seawater."
Reason:
- While it is correct that a thermonuclear fusion reactor is better than a fission reactor for several reasons, including higher energy release and less radioactive waste production, the availability of fuel from seawater is not accurate.
- Fusion reactions require isotopes of hydrogen, such as deuterium and tritium, as fuel. Deuterium can be extracted from seawater, but tritium is a radioactive isotope that is not naturally abundant and needs to be produced artificially.
- Tritium can be produced by exposing lithium to neutron radiation, which can be generated by a fission reactor or a fusion reactor itself. However, the process of producing tritium is not as straightforward as extracting deuterium from seawater.
- Tritium is also highly radioactive and has a short half-life, which means it requires careful handling and containment. It cannot be easily stored or transported.
- Therefore, the fuel required for fusion reactions is not readily available in abundance from seawater, as stated in option B.
Correct statements:
a) For the same mass of substances involved, a fusion reaction releases much more energy than a fission reaction.
- This is true. Fusion reactions release a tremendous amount of energy, several times more than fission reactions. The fusion of hydrogen atoms into helium is the same process occurring in the Sun and other stars, which produces immense amounts of energy.
c) A fusion reaction can be much more easily controlled than a fission reaction.
- This is true. Fusion reactions require extremely high temperatures and pressures to sustain, and if these conditions are not maintained, the reaction will cease. This inherent stability makes fusion reactions more easily controllable than fission reactions, which can lead to runaway chain reactions if not properly regulated.
d) A fusion reaction produces almost no radioactive waste.
- This is true. Fusion reactions do not produce long-lived radioactive waste like fission reactions. The only radioactive byproduct of fusion is tritium, which has a relatively short half-life and can be managed safely.
In summary, option B is the wrong statement because the fuel required for fusion reactions is not readily available in abundance from seawater.
The disintegration energy is- a)the difference between the initial energy and the total energy of the decay products
- b)the difference between the initial mass and the total mass of the decay products
- c)the difference between the initial mass energy and the total mass energy of the decay products
- d)none of the above
Correct answer is option 'C'. Can you explain this answer?
The disintegration energy is
a)
the difference between the initial energy and the total energy of the decay products
b)
the difference between the initial mass and the total mass of the decay products
c)
the difference between the initial mass energy and the total mass energy of the decay products
d)
none of the above
|
|
Rakibul Halsana answered |
CCCC
The control rods in a nuclear reactor- a)Absorb neutrons
- b)Accelerate neutrons
- c)Slow down neutrons
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
The control rods in a nuclear reactor
a)
Absorb neutrons
b)
Accelerate neutrons
c)
Slow down neutrons
d)
None of the above
|
|
Shreya Gupta answered |
Control rods are used in nuclear reactors to control the fission rate of uranium and plutonium. They are composed of chemical elements such as boron, silver, indium and cadmium that are capable of absorbing many neutrons without themselves fissioning.
Which of the following particles can be added to the nucleus of an atom without changing its chemical properties?- a)Alpha Particles
- b)Protons
- c)Neutrons
- d)Electrons
Correct answer is option 'C'. Can you explain this answer?
Which of the following particles can be added to the nucleus of an atom without changing its chemical properties?
a)
Alpha Particles
b)
Protons
c)
Neutrons
d)
Electrons
|
|
Rajat Kapoor answered |
Adding a neutron to the nucleus will make no change in the chemical properties of the atom. The atom will have the same number of protons and therefore the same number of electrons. It is the number of electrons that determines chemical properties.
Actually, with hydrogen the addition of a neutron will almost double its mass and thus cause it to behave a little differently chemically.
Of course if the added neutron causes the nucleus to fission, decay, or otherwise change, that will change the chemistry.
A fusion bomb involves:- a)breaking of a heavy nucleus into a lighter ones
- b)explosion of TNT
- c)synthesis of lighter nuclei into heavier ones
- d)burning of huge amount of coal
Correct answer is option 'C'. Can you explain this answer?
A fusion bomb involves:
a)
breaking of a heavy nucleus into a lighter ones
b)
explosion of TNT
c)
synthesis of lighter nuclei into heavier ones
d)
burning of huge amount of coal

|
Akshay Shah answered |
An atomic bomb, by contrast, uses the energy released when a heavy atomic nucleus splits, or fissions, into two lighter nuclei. ... The energy thus produced forms the explosive power of a hydrogen bomb. Deuterium and tritium, which are isotopes of hydrogen, provide ideal interacting nuclei for the fusion process.
When a hydrogen bomb explodes, which of the following is used?- a)fission
- b)both
- c)neither of two
- d)fusion
Correct answer is option 'B'. Can you explain this answer?
When a hydrogen bomb explodes, which of the following is used?
a)
fission
b)
both
c)
neither of two
d)
fusion
|
|
Naina Bansal answered |
Hydrogen bomb or H-bomb, weapon deriving a large portion of its energy from the nuclear fusion of hydrogen isotopes. In an atomic bomb, uranium or plutonium is split into lighter elements that together weigh less than the original atoms, the remainder of the mass appearing as energy. Unlike this fission bomb, the hydrogen bomb functions by the fusion, or joining together, of lighter elements into heavier elements. The end product again weighs less than its components, the difference once more appearing as energy. Because extremely high temperatures are required in order to initiate fusion reactions, the hydrogen bomb is also known as a thermonuclear bomb.
Why does the fusion occur at high temperature?- a)kinetic energy is high enough to overcome repulsion between nuclei.
- b)nuclei break up at high temperature
- c)atoms are ionised at high temperature
- d)molecules break up at high temperature
Correct answer is option 'A'. Can you explain this answer?
Why does the fusion occur at high temperature?
a)
kinetic energy is high enough to overcome repulsion between nuclei.
b)
nuclei break up at high temperature
c)
atoms are ionised at high temperature
d)
molecules break up at high temperature
|
|
Arun Khanna answered |
The high temperature gives the hydrogen atoms enough energy to overcome the electrical repulsion between the protons. Fusion requires temperatures about 100 million Kelvin (approximately six times hotter than the sun's core). At these temperatures, hydrogen is a plasma, not a gas.
β -rays and γ-rays are respectively- a)neutrons and electromagnetic radiation of wavelengths shorter than X-rays
- b)protons and neutrons of wavelengths shorter than X-rays
- c)protons and electromagnetic radiation of wavelengths shorter than X-rays
- d)electrons and electromagnetic radiation of wavelengths shorter than X-rays
Correct answer is option 'D'. Can you explain this answer?
β -rays and γ-rays are respectively
a)
neutrons and electromagnetic radiation of wavelengths shorter than X-rays
b)
protons and neutrons of wavelengths shorter than X-rays
c)
protons and electromagnetic radiation of wavelengths shorter than X-rays
d)
electrons and electromagnetic radiation of wavelengths shorter than X-rays
|
|
Divyansh Kulkarni answered |
Beta radiation ~ Stream of electrons (unit negative charge). Beta positive radiation is when a positron is emitted rather than an electron.
Gamma radiation ~ Electromagnetic radiation of very short wavelength = high photon energy.
Gamma radiation ~ Electromagnetic radiation of very short wavelength = high photon energy.
A free neutron decays into- a)a proton, a positron and a antineutrino
- b)a proton, a positron and a neutrino
- c)a proton, an electron and an antineutrino
- d)a proton, an electron and a neutrino
Correct answer is option 'C'. Can you explain this answer?
A free neutron decays into
a)
a proton, a positron and a antineutrino
b)
a proton, a positron and a neutrino
c)
a proton, an electron and an antineutrino
d)
a proton, an electron and a neutrino
|
|
Rajeev Saxena answered |
The decay of free neutrons is energy feasible because the mass of a neutron is greater than the sum of the masses of the proton and electron it decays into. But where a neutron is paired with a proton its decay is not energy feasible and thus such neutrons within nuclei are stable.
Binding energy per nucleon is the ratio of- a)the binding energy of the nucleus to the number of nucleons in that nucleus.
- b)energy required to remove a nucleon to atomic weight
- c)binding energy of a nucleon to the atomic number
- d)binding energy of a nucleus to the atomic number
Correct answer is option 'A'. Can you explain this answer?
Binding energy per nucleon is the ratio of
a)
the binding energy of the nucleus to the number of nucleons in that nucleus.
b)
energy required to remove a nucleon to atomic weight
c)
binding energy of a nucleon to the atomic number
d)
binding energy of a nucleus to the atomic number
|
|
Anjana Sharma answered |
Binding energy per nucleon is the ratio of the binding energy of a nucleus to the number of the nucleons.
Binding energy per nucleon = (Total binding energy) / (Number of nucleon)
Measure of stability of the nucleus: Larger the binding energy per nucleon, the greater the work that must be done to remove the nucleon from the nucleus, the more stable the nucleus.
When a hydrogen bomb explodes, which of the following is used?- a)fission
- b)fusion
- c)neither of two
- d)both
Correct answer is option 'D'. Can you explain this answer?
When a hydrogen bomb explodes, which of the following is used?
a)
fission
b)
fusion
c)
neither of two
d)
both

|
Dr Manju Sen answered |
The hydrogen bomb is a nuclear weapon that uses a mixture of fission and fusion to produce a massive explosion.
The nuclear fission generates enough heat to initiate the nuclear fusion reaction. After that, the nuclear fusion releases enormous amounts of energy, making the hydrogen bomb a lot more powerful than an atomic bomb.
The nuclear fission generates enough heat to initiate the nuclear fusion reaction. After that, the nuclear fusion releases enormous amounts of energy, making the hydrogen bomb a lot more powerful than an atomic bomb.
Nuclear forces are- a)spin dependent and have no non-central part
- b)spin dependent and have a non-central part
- c)spin independent and have no non-central part
- d)spin independent and have a non-central part
Correct answer is option 'D'. Can you explain this answer?
Nuclear forces are
a)
spin dependent and have no non-central part
b)
spin dependent and have a non-central part
c)
spin independent and have no non-central part
d)
spin independent and have a non-central part

|
EduRev JEE answered |
Nuclear forces are fundamental interactions that govern the behaviour of protons and neutrons within an atomic nucleus. Here are some key characteristics:
- The nuclear force is spin independent, meaning it does not rely on the spin orientation of nucleons.
- It has a non-central part, which implies that the force varies with the spatial arrangement of nucleons.
- This force is significantly stronger than the Coulomb force, which acts between charged particles.
- The nuclear force operates over a very short range, typically around a few femtometres (fm).
In summary, the nuclear force is essential for holding the nucleus together, overcoming the repulsive forces between positively charged protons, and ensuring the stability of atomic nuclei.
Chapter doubts & questions for Nuclei - Online MCQ Tests for JEE 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Nuclei - Online MCQ Tests for JEE in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup