All Exams >
NEET >
Chemistry Class 11 >
All Questions
All questions of Chemical Bonding and Molecular Structure for NEET Exam
Hybridisation of Acetylene is- a)sp
- b)sp2
- c)sp3
- d)dsp2
Correct answer is option 'A'. Can you explain this answer?
Hybridisation of Acetylene is
a)
sp
b)
sp2
c)
sp3
d)
dsp2
|
|
Riya Banerjee answered |
Since acetylene is made up of triple bond. So the hybridization of carbon in acetylene is sp.
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Assuming that Hund's rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is
- a)1 and diamagnetic
- b)0 and diamagnetic
- c)1 and paramagnetic
- d)0 and paramagnetic
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Assuming that Hund's rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is
a)
1 and diamagnetic
b)
0 and diamagnetic
c)
1 and paramagnetic
d)
0 and paramagnetic
|
|
Anjana Sharma answered |
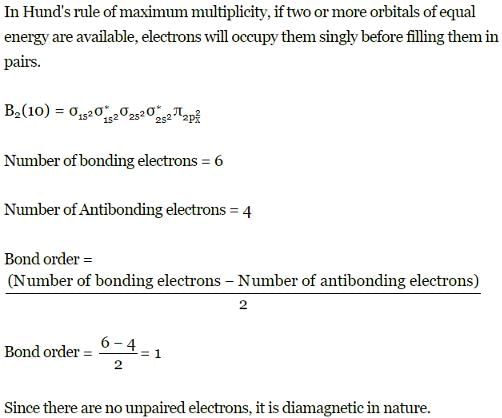
Which of the following is/are correct statement(s)?a)s + py→ two sp-hybrid orbitals lying in the yz planeb)s + px → two sp-hybrid orbitals lying in xz planec)(s + px + pz) → three sp2-hybrid orbitals lying in xz planed)(s + py) → two sp-hybrid orbitals lying along y-axisCorrect answer is option 'C,D'. Can you explain this answer?
|
|
Geetika Shah answered |
Incorrect (b)
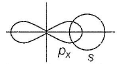
two sp-hybrid orbitals are along x-axis.
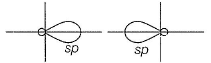
two sp-hybrid orbitals are along x-axis.
A pi-bond is formed by the overlap of:- a)p-p orbitals in sidewise manner
- b)s-p orbitals
- c)s-s orbitals
- d)p-p orbitals in end to end fashion
Correct answer is option 'A'. Can you explain this answer?
A pi-bond is formed by the overlap of:
a)
p-p orbitals in sidewise manner
b)
s-p orbitals
c)
s-s orbitals
d)
p-p orbitals in end to end fashion
|
|
Raghav Bansal answered |
pi-bond is always formed by sidewise overlapping of p – p orbitals in sidewise manner.
Bond order of 1.5 is shown by : [2012] - a)O2+
- b)O2-
- c)O22+
- d)O2
Correct answer is option 'B'. Can you explain this answer?
Bond order of 1.5 is shown by : [2012]
a)
O2+
b)
O2-
c)
O22+
d)
O2
|
|
Rajat Kapoor answered |
N₂=N+N = 7e⁻+7e⁻ = 14e- which has bond order =3
O₂=8e⁻+8e⁻ = 16e⁻ which has bond order = 2
O₂⁺=8e⁻+8e⁻ - 1e⁻ = 15e⁻ which has bond order= 2.5
O₂⁻ = 8e⁻ +8e⁻ +1e⁻ =17 e⁻ which has bond order=1.5
So option B is correct .
Which of the following compounds has a 3-centre bond?[1996]- a)Diborane
- b)Carbon dioxide
- c)Boron trifluroide
- d)Ammonia
Correct answer is option 'A'. Can you explain this answer?
Which of the following compounds has a 3-centre bond?[1996]
a)
Diborane
b)
Carbon dioxide
c)
Boron trifluroide
d)
Ammonia

|
Krish Saha answered |
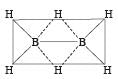
The bond represented by dots form the 3-centred electron pair bond. The idea of three centred electron pair bond B–H–B bridges is necessitated because diborane does not have sufficient electrons to form normal covalent bonds. It has only 12 electrons instead of 14 required to give simple ethane like structure for diborane.
Valence Bond Theory was developed in the year- a)1916
- b)1927
- c)1930
- d)1932
Correct answer is option 'B'. Can you explain this answer?
Valence Bond Theory was developed in the year
a)
1916
b)
1927
c)
1930
d)
1932
|
|
Krishna Iyer answered |
The valence bond (VB) theory of bonding was mainly developed by Walter Heitler and Fritz London in 1927, and later modified by Linus Pauling to take bond direction into account. The VB approach concentrates on forming bonds in localized orbitals between pairs of atoms, and hence retains the simple idea of Lewis structures and electron pairs.
Expansion of octet can not take place in- a)N
- b)S
- c)Si
- d)P
Correct answer is option 'A'. Can you explain this answer?
Expansion of octet can not take place in
a)
N
b)
S
c)
Si
d)
P

|
Harshad Nair answered |
N(7) = 1s22s22p3
Nitrogen does not have (2d) orbitals. Thus, (more than 8) electrons cannot be accomodated in second orbit.
Nitrogen does not have (2d) orbitals. Thus, (more than 8) electrons cannot be accomodated in second orbit.
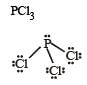
The shape of the below molecule is
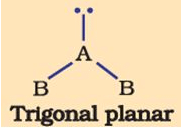
- a)trigonal
- b)rigonal planar
- c)see saw
- d)bent
Correct answer is option 'D'. Can you explain this answer?
The shape of the below molecule is

a)
trigonal
b)
rigonal planar
c)
see saw
d)
bent
|
|
Lavanya Menon answered |
The order of Repulsion: lone pair-lone pair > lone pair-bond pair > bond pair-bond pair
Due to the extra lone pair electron, the shape becomes bent.
Pick out the pair of species having identical shapes for both the molecules.- a)BF3 , PCl3
- b)PF , IF5
- c)CF4,SF4
- d)XeF2 , CO2
Correct answer is 'D'. Can you explain this answer?
Pick out the pair of species having identical shapes for both the molecules.
a)
BF3 , PCl3
b)
PF , IF5
c)
CF4,SF4
d)
XeF2 , CO2
|
|
Raghav Bansal answered |
Both XeF2 & CO2 have linear structures. Both are actually linear Tri-atomic molecules.
Which molecule has a trigonal pyramidal shape?- a)AB4E
- b)AB2E2
- c)AB3E
- d)AB2E
Correct answer is option 'C'. Can you explain this answer?
Which molecule has a trigonal pyramidal shape?
a)
AB4E
b)
AB2E2
c)
AB3E
d)
AB2E
|
|
Geetika Shah answered |
AB3E: trigonal pyramidal (central atom + 3 outer atoms make a pyramid)
→ start with AB4 molecule (tetrahedral) and replace a B atom w/ lone pair
→ lone pair electrons push bonding electrons away
→ bond angles are now less than 109.5°
→ start with AB4 molecule (tetrahedral) and replace a B atom w/ lone pair
→ lone pair electrons push bonding electrons away
→ bond angles are now less than 109.5°
N2 and O2 are converted into monocations, N2+ and O2+ respectively. Which of the following statements is wrong ? [1997]- a)In N2, the N—N bond weakens
- b)In O2, the O—O bond order increases
- c)In O2, paramagnetism decreases
- d)N2+ becomes diamagnetic
Correct answer is option 'B'. Can you explain this answer?
N2 and O2 are converted into monocations, N2+ and O2+ respectively. Which of the following statements is wrong ? [1997]
a)
In N2, the N—N bond weakens
b)
In O2, the O—O bond order increases
c)
In O2, paramagnetism decreases
d)
N2+ becomes diamagnetic

|
Rohan Unni answered |
We know that in O2 bond, the order is 2 and in O2– bond, the order is 1.5. Therefore the wrong statements is (b)
Which of the following angle corresponds to sp2 hybridisation?a)120∘b)180∘c)90∘d)109∘Correct answer is option 'A'. Can you explain this answer?
|
|
Samridhi Pillai answered |
sp2 hybridisation gives three sp2 hybrid orbitals which are planar triangular forming an angle of 120° with each other.
The electronic configurations of three elements A, B and C are given below.
Answer the questions from 14 to 17 on the basis of these configurations.
A ls22s22p6
B ls22s22p63s23p3
C ls22s22p63s23p
The electronic configurations of three elements A, B and C are given below.
Answer the questions from 14 to 17 on the basis of these configurations.
A ls22s22p6
B ls22s22p63s23p3
C ls22s22p63s23p
bond lengths are lower in elements havinga)crystal structureb)double bondc)triple bondd)single bondCorrect answer is option 'C'. Can you explain this answer?
|
|
Rajesh Gupta answered |
Single bond has higher bond length than multiple bond.
Which of the following has pπ – dπ bonding?- a)NO3–
- b)SO32– [2002]
- c)BO33–
- d)CO32–
Correct answer is option 'B'. Can you explain this answer?
Which of the following has pπ – dπ bonding?
a)
NO3–
b)
SO32– [2002]
c)
BO33–
d)
CO32–

|
Smruti Sucharita answered |
Answer is b bcoz Sulphur has d orbital...
Q. Which of the following species contain at least one atom that violates the octet rule?a)O— Cl—-Ob)F— Xe— Fc)PCI5d)SF6Correct answer is option 'A,B,C,D'. Can you explain this answer?

|
Knowledge Hub answered |
(a) Octet of Cl expanded to (9).
(b) Octet Xe expanded to (10).
(c) Octet of P expanded to (10).
(b) Octet Xe expanded to (10).
(c) Octet of P expanded to (10).
Which of the following molecule doesn’t have a lone pair?- a)BeCl2
- b)XeF4
- c)NH3
- d)H2O
Correct answer is option 'A'. Can you explain this answer?
Which of the following molecule doesn’t have a lone pair?
a)
BeCl2
b)
XeF4
c)
NH3
d)
H2O
|
|
Raghav Bansal answered |
BeCl2 has no lone pairs on the beryllium. Thus, the electrons on the chlorides willtry to stay far apart from each other, since their corresponding electrons repel each other (while experiencing no deflection from electrons on a central atom). Thus themolecule is linear in shape.
In BrF3 molecule, the lone pairs occupy equatorial positions to minimize [2004]- a)lone pair - bond pair repulsion only
- b)bond pair - bond pair repulsion only
- c)lone pair - lone pair repulsion and lone pair -bond pair repulsion
- d)lone pair - lone pair repulsion only
Correct answer is option 'C'. Can you explain this answer?
In BrF3 molecule, the lone pairs occupy equatorial positions to minimize [2004]
a)
lone pair - bond pair repulsion only
b)
bond pair - bond pair repulsion only
c)
lone pair - lone pair repulsion and lone pair -bond pair repulsion
d)
lone pair - lone pair repulsion only

|
Abhishek Choudhary answered |
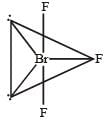
In BrF3, both bond pairs as well as lone pairs of electrons are present. Due to the presence of lone pairs of electrons (lp) in the valence shell, the bond angle is contracted and the molecule takes the T-shape. This is due to greater repulsion between two lone pairs or between a lone pair and a bond pair than between the two bond pairs.
Direction (Q. Nos. 13-16) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q.
In which of the following I is more volatile than II?
- a)
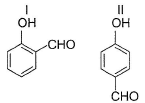
- b)
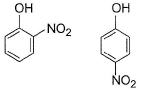
- c)

- d)
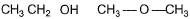
Correct answer is option 'A,B'. Can you explain this answer?
Direction (Q. Nos. 13-16) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q.
In which of the following I is more volatile than II?
In which of the following I is more volatile than II?
a)
b)
c)
d)
|
|
Hansa Sharma answered |
I. Intermolecular H-bonding makes boiling point higher than that of I. Thus, I is more volatile
II. Ortho nitrophenol is more volatile than para nitrophenol because O-Nitrophenol has intramolecular hydrogen bonding whereas para nitrophenol has inter molecular H bonding and so boils relatively at higher temperature
(c) BP of H2O > > H2S
(d) BP of CH3CH2OH (due to H-bonding) > > CH3— O — CH3
A qualitative measure of the stability of an ionic compound is provided- a)ionization enthalpy
- b)lattice enthalpy
- c)Electron affinity
- d)electron gain enthalpy
Correct answer is option 'B'. Can you explain this answer?
A qualitative measure of the stability of an ionic compound is provided
a)
ionization enthalpy
b)
lattice enthalpy
c)
Electron affinity
d)
electron gain enthalpy

|
Sounak Chaudhary answered |
stability of ionic bond is directly propotional to lattice energy.
Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3 . The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is :
- a)HF > H2O > NH3
- b)H2O > HF > NH3
- c)NH3 > HF > H2O
- d)NH3 > H2O > HF
Correct answer is option 'B'. Can you explain this answer?
Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3 . The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is :
a)
HF > H2O > NH3
b)
H2O > HF > NH3
c)
NH3 > HF > H2O
d)
NH3 > H2O > HF
|
|
Neha Joshi answered |
H2O>HF>NH3
Strength of hydrogen bonding depends on the size and electronegativity of the atom.
Smaller the size of the atom, greater is the electronegativity and hence stronger is the H−bonding. Thus, the order of strength of H-bonding is H...F>H...O>H...N.
But each HF molecule is linked only to two other HF molecules while each H2O molecule is linked to four other H2O molecules through H−bonding.
Hence, the decreasing order of boiling points is H2O>HF>NH3.
Valence bond theory was introduced by:
- a)Gillespie
- b)Heitler
- c)Pauling
- d)Lewis
Correct answer is option 'B'. Can you explain this answer?
Valence bond theory was introduced by:
a)
Gillespie
b)
Heitler
c)
Pauling
d)
Lewis
|
|
Preeti Iyer answered |
He then called up his associate Fritz London and they worked out the details of the theory over the course of the night. Later, Linus Pauling used the pair bonding ideas of Lewis together with Heitler–London theory to develop two other key concepts in VB theory: resonance (1928) and orbital hybridization (1930).
Rank the following bonds in order of increasing polarity: H-N, H-O, H-C.- a)H-O < H-N < H-C
- b)H - C < H-N < H-O.
- c)H - C < H-O < H-N.
- d)H-N < H-O < H-C
Correct answer is option 'B'. Can you explain this answer?
Rank the following bonds in order of increasing polarity: H-N, H-O, H-C.
a)
H-O < H-N < H-C
b)
H - C < H-N < H-O.
c)
H - C < H-O < H-N.
d)
H-N < H-O < H-C

|
Rahul Chaudhary answered |
O is more electronegative than N which is more electronegative than C.
Which one of the following arrangements represents the increasing bond orders of the given species? [1999]- a)NO+ < NO < NO– < O2–
- b)O2– < NO– < NO <NO+
- c)NO– < O2– < NO < NO+
- d)NO < NO+ < O2– < NO–
Correct answer is option 'B'. Can you explain this answer?
Which one of the following arrangements represents the increasing bond orders of the given species? [1999]
a)
NO+ < NO < NO– < O2–
b)
O2– < NO– < NO <NO+
c)
NO– < O2– < NO < NO+
d)
NO < NO+ < O2– < NO–
|
|
Mira Joshi answered |

Bond order of NO+ =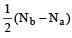
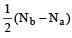
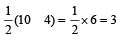
Similarly, Bond order of NO = 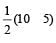
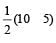
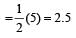
Bond order of NO– 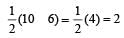
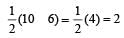
Bon d order of 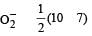
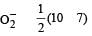
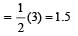
By above calculation, we get Decreasing bond order NO+ > NO > NO– > O-2
In a regular octahedral molecule, MX6 the number of X - M - X bonds at 180° is [2004]- a)three
- b)two
- c)six
- d)four
Correct answer is option 'A'. Can you explain this answer?
In a regular octahedral molecule, MX6 the number of X - M - X bonds at 180° is [2004]
a)
three
b)
two
c)
six
d)
four
|
|
Lavanya Menon answered |
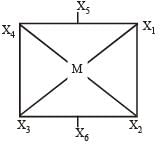
Thus here bond angles between X4 - M - X2 = 180°
X1 - M - X3 = 180°
X5 - M - X6 = 180°
X1 - M - X3 = 180°
X5 - M - X6 = 180°
In which one of the following species the central atom has the type of hybridisation which si not the same as that present in the other three? - a)SF4
- b)I3-
- c)SbCl52-
- d)PCl5
Correct answer is option 'C'. Can you explain this answer?
In which one of the following species the central atom has the type of hybridisation which si not the same as that present in the other three?
a)
SF4
b)
I3-
c)
SbCl52-
d)
PCl5
|
|
Geetika Shah answered |
Molecules having the same number of hybrid orbitals, have same hybridisation and number of hybrid oebitals.
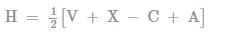
where,
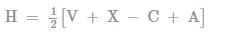
where,
V= number of valance electrons of central atom
X = number of monovalent atoms
C= charge on cation
A = charge on anion
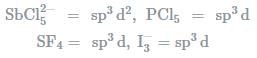
Select the correct statement(s) about NO2.- a)It is paramagnetic in nature
- b)It forms dimer and paramagnetic is lost
- c)NO2 and dimer formed have sp2-hybridised N-atom
- d)Brown colour of NO2 fades and (N— N) bond length is greater than normal (N— N) bond length
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Select the correct statement(s) about NO2.
a)
It is paramagnetic in nature
b)
It forms dimer and paramagnetic is lost
c)
NO2 and dimer formed have sp2-hybridised N-atom
d)
Brown colour of NO2 fades and (N— N) bond length is greater than normal (N— N) bond length
|
|
Pooja Shah answered |
Due to unpaired electron paramagnetic, N-atom in NO2 is electron deficie nt thus, to complete octet, dimer is formed.
In N2O4 formation, each N-atom gets
Thus, (a), (b), (c) and (d) are correct.
Mean bond enthalpy of different bonds are given
 Out of the given pairs, which compound is more stable than the other?
Out of the given pairs, which compound is more stable than the other?
- a)
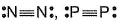
- b)
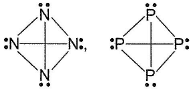
- c)
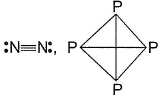
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Mean bond enthalpy of different bonds are given
Out of the given pairs, which compound is more stable than the other?
a)
b)
c)
d)
None of these

|
Knowledge Hub answered |
Bond enthalpy of six (P— P) bond = 6 x 215 = 1290 k j mol-1
Bond enthalpy of six (N — N) bond = 6 x 160
= 960 kJ mol-1
Thus, P4 is more stable than N4.
Hybridization of C2and C3of H3C −− CH = C = CH −− CH3are - a)Sp, Sp3
- b)Sp2, Sp2
- c)Sp2, Sp
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
Hybridization of C2and C3of H3C −− CH = C = CH −− CH3are
a)
Sp, Sp3
b)
Sp2, Sp2
c)
Sp2, Sp
d)
None of the above
|
|
Arun Khanna answered |
The first carbon atom forms four sigma bonds, three with Hydrogen and one with carbon. So, the carbon here is sp3 hybridised.The second carbon atom forms three sigma bonds and one pi bond. The three sigma bonds can be possible only when carbon is sp2 hybridised. The fourth electron forms a pi overlap with an electron from third carbon atom.
Carbon atom 3 forms two sigma bonds and is sp hybridised. The two p electrons form a pi bond with p electrons of the neighbouring carbon atoms.Carbon atom 4 is similar to carbon 2, forms 3 sigma bonds and is sp2 hybridised.
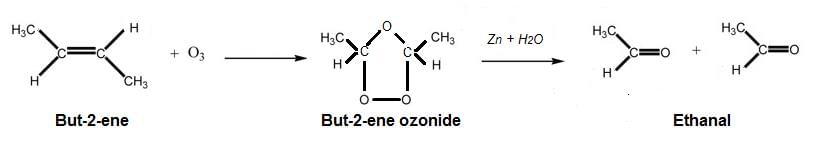
As the aldehyde formed has molar mass of 44u, so the aldehyde is acetaldehyde or ethanal. The alkene that gives rise to ethanal, is but-2-ene. Ozonolysis leads to breaking the alkene molecule into two molecules at the double bond. As only one product, ethanal is formed, two carbon atoms surround the two sides of the double bond.
Which molecule has a bent shape?- a)CO2
- b)BeH2
- c)H2O
- d)NF3
Correct answer is option 'C'. Can you explain this answer?
Which molecule has a bent shape?
a)
CO2
b)
BeH2
c)
H2O
d)
NF3
|
|
Shreya Singh answered |
Oxygen is sp3 hybridised in H2O molecule..... and it should have tetrahedral structure with bond angle 109•28'...but The oxygen has 6 valence electrons and thus needs 2 more electrons from 2 hydrogen atoms to complete its octet. This then leaves two lone electron pairs that are not bonded to any other atoms. ....The 2 lone electron pairs exerts a little extra repulsion on the two bonding hydrogen atoms to create a slight compression to a 104obond angle......and because of this it acquires bent/v/angular shape..
Covalency of carbon in CO is three because- a)an unexcited carbon atom has two unpaired electrons
- b)the C-atom can be an acceptor of an electron pair
- c)the C-atom has four valence electrons
- d)the maximum covalency of carbon is three
Correct answer is option 'B'. Can you explain this answer?
Covalency of carbon in CO is three because
a)
an unexcited carbon atom has two unpaired electrons
b)
the C-atom can be an acceptor of an electron pair
c)
the C-atom has four valence electrons
d)
the maximum covalency of carbon is three
|
|
Krishna Iyer answered |
In structure I, octet of oxygen is complete but octet of carbon is incomplete. Hence, carbon is an acceptor of an electron pair from oxygen. Thus, covalency of carbon is three in CO.
The relationship between the dissociation energy of N2 and N2+ is : [2000]- a)Dissociation energy of N2+ > dissociation energy of N2
- b)Dissociation energy of N2 = dissociation energy of N2+
- c)Dissociation energy of N2 > dissociation energy of N2+
- d)Dissociation energy of N2 can either be lower or higher than the dissociation energy of N2+
Correct answer is option 'C'. Can you explain this answer?
The relationship between the dissociation energy of N2 and N2+ is : [2000]
a)
Dissociation energy of N2+ > dissociation energy of N2
b)
Dissociation energy of N2 = dissociation energy of N2+
c)
Dissociation energy of N2 > dissociation energy of N2+
d)
Dissociation energy of N2 can either be lower or higher than the dissociation energy of N2+

|
Mahi Shah answered |
Dissociation energy of any molecules depends upon bond order. Bond order in N2 molecule is 3 while bond order in N+2 is 2.5.
Further we know that more the Bond order, more is the stability and more is the BDE.
Further we know that more the Bond order, more is the stability and more is the BDE.
The number of types of bonds between two carbon atoms in calcium carbide is- a)Two sigma, two pi
- b)One sigma, two pi
- c)One sigma, one pi
- d)Two sigma, one pi
Correct answer is option 'B'. Can you explain this answer?
The number of types of bonds between two carbon atoms in calcium carbide is
a)
Two sigma, two pi
b)
One sigma, two pi
c)
One sigma, one pi
d)
Two sigma, one pi
|
|
Jyoti Kumar answered |
Explanation:
Calcium carbide is a chemical compound with the chemical formula CaC2. It is composed of calcium and two carbon atoms. The two carbon atoms in calcium carbide are bonded together, and the type of bond between them is determined by the way they share electrons.
The types of bonds between two carbon atoms in calcium carbide are:
1. Sigma Bond: A sigma bond is formed when two atoms overlap their atomic orbitals end to end, creating a single bond. In calcium carbide, there is one sigma bond between the two carbon atoms.
2. Pi Bond: A pi bond is formed when two atoms share electrons in parallel orbitals that overlap above and below the internuclear axis. In calcium carbide, there are two pi bonds between the two carbon atoms.
Therefore, the correct answer is option B, which is one sigma bond and two pi bonds between the two carbon atoms in calcium carbide.
Calcium carbide is a chemical compound with the chemical formula CaC2. It is composed of calcium and two carbon atoms. The two carbon atoms in calcium carbide are bonded together, and the type of bond between them is determined by the way they share electrons.
The types of bonds between two carbon atoms in calcium carbide are:
1. Sigma Bond: A sigma bond is formed when two atoms overlap their atomic orbitals end to end, creating a single bond. In calcium carbide, there is one sigma bond between the two carbon atoms.
2. Pi Bond: A pi bond is formed when two atoms share electrons in parallel orbitals that overlap above and below the internuclear axis. In calcium carbide, there are two pi bonds between the two carbon atoms.
Therefore, the correct answer is option B, which is one sigma bond and two pi bonds between the two carbon atoms in calcium carbide.
Which of the following would have a permanent dipolemoment? [2005]- a)SiF4
- b)SF4
- c)XeF4
- d)BF3
Correct answer is option 'B'. Can you explain this answer?
Which of the following would have a permanent dipolemoment? [2005]
a)
SiF4
b)
SF4
c)
XeF4
d)
BF3

|
Prasenjit Pillai answered |
SF4 has permanent dipole moment.
SF4 has sp3d hybridization and see saw shape (irregular geometry).
SF4 has sp3d hybridization and see saw shape (irregular geometry).
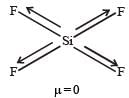
Whereas XeF4 shows squre planar geometry SiF4 has tetrahedral shape and BF3 has Trigonal planar shape. All these are symmetric molecules. Hence μ ≠ 0.
Among the following species linear shape is found in:- a)SO2
- b)O3
- c)NO2+
- d)NO2–
Correct answer is option 'C'. Can you explain this answer?
Among the following species linear shape is found in:
a)
SO2
b)
O3
c)
NO2+
d)
NO2–
|
|
Rohit Shah answered |
In NO2(+) i.e. nitronium ion N-atom has sp-hybridisation ; so, it adopts linear geometry & O-N-O bond angle is 180 deg.
While, in NO2(–) i.e. nitrite ion, N–atom has sp2 hybridisation; so, it adopts bent geometry, for NO2–, actual O-N-O bond angle is 115 deg (yes, it's slightly deviated from expected 120deg because of repulsion between the interacting bond pairs and lone pair of electrons.
Two atoms are said to be bonded when:- a)System acquires minimum energy
- b)Potential energy becomes maximum
- c)Magnitude of attractive forces is greater than repulsive forces
- d)Energy becomes zero
Correct answer is option 'A'. Can you explain this answer?
Two atoms are said to be bonded when:
a)
System acquires minimum energy
b)
Potential energy becomes maximum
c)
Magnitude of attractive forces is greater than repulsive forces
d)
Energy becomes zero
|
|
Anjana Sharma answered |
Attractive forces tend to bring two atoms close to each other whereas repulsive forces tend to move them away. In hydrogen, the magnitude of the new attractive forces is greater than that of new repulsive forces. As a result, two atoms come close to each other and potential energy decreases. The atoms approach each other until the equilibrium stage is reached where the net force of attraction balances the force of repulsion and system acquires minimum energy.
When a gas phase atom in its ground state gains an electron. This is called- a)electron gain enthalpy
- b)ionization enthalpy
- c)Electron affinity
- d)lattice enthalpy
Correct answer is option 'A'. Can you explain this answer?
When a gas phase atom in its ground state gains an electron. This is called
a)
electron gain enthalpy
b)
ionization enthalpy
c)
Electron affinity
d)
lattice enthalpy
|
|
Pooja Shah answered |
Electron gain enthalpy is the energy released when an electron is gained by an isolated gaseous atom to form an isolated gaseous anion.
Among the following molecules, the molecule with trigonal planar geometry is:- a)BF3
- b)IF3
- c)NH3
- d)PCl3
Correct answer is option 'A'. Can you explain this answer?
Among the following molecules, the molecule with trigonal planar geometry is:
a)
BF3
b)
IF3
c)
NH3
d)
PCl3
|
|
Gaurav Kumar answered |
(a) BF₃ ⇒ 3bp + 0/p ⇒ sp³ -hybridisation and triagonal planar geometry.
(b) IF₃ ⇒ 3bp + 2/p ⇒ sp³d -hybridisation and T shape.
(c) NH₃ ⇒ 3bp + 1/p ⇒ sp³ -hybridisation and pyraminal geometry.
(d) PH₃ ⇒ 3bp + 1/p ⇒ sp³ -hybridisation and pyraminal geometry.
(b) IF₃ ⇒ 3bp + 2/p ⇒ sp³d -hybridisation and T shape.
(c) NH₃ ⇒ 3bp + 1/p ⇒ sp³ -hybridisation and pyraminal geometry.
(d) PH₃ ⇒ 3bp + 1/p ⇒ sp³ -hybridisation and pyraminal geometry.
H.O.H bond angle in water is- a)1100
- b)2400
- c)1040
- d)4160
Correct answer is option 'C'. Can you explain this answer?
H.O.H bond angle in water is
a)
1100
b)
2400
c)
1040
d)
4160

|
Aravind Saha answered |
Due to presence of two lone pairs on O in H2O bond angle reduce to 104 from 109.
Considering x-axis as the internuclear axis, which out of the following will not form sigma bond.- a)1s and 1s
- b)1s and 2px
- c)2py and 2py
- d)1s and 2s
Correct answer is option 'C'. Can you explain this answer?
Considering x-axis as the internuclear axis, which out of the following will not form sigma bond.
a)
1s and 1s
b)
1s and 2px
c)
2py and 2py
d)
1s and 2s
|
|
Anjana Sharma answered |
Sigma bond is always formed between two half-filled atomic orbitals along their internuclear axis.i.e the line joining the centres of the nuclei of two atoms(axial overlapping). 2py and 2py will not form a sigma bond because taking x-axis as the internuclear axis, there will be lateral (sideway) overlap between the two 2pv orbitals forming a straight pi bond.
The H-bond is shortest in
- a)S— H---S
- b)N— H ... O
- c)F— H ... F
- d)F— H ... O
Correct answer is option 'C'. Can you explain this answer?
The H-bond is shortest in
a)
S— H---S
b)
N— H ... O
c)
F— H ... F
d)
F— H ... O
|
|
Arjun Singhania answered |
A hydrogen bond is a weak type of force that forms a special type of dipole-dipole attraction which occurs when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons.
F is the most electronegative atom. Hence hydrogen bond is shortest in F - H ........F.
F is the most electronegative atom. Hence hydrogen bond is shortest in F - H ........F.
Chapter doubts & questions for Chemical Bonding and Molecular Structure - Chemistry Class 11 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Bonding and Molecular Structure - Chemistry Class 11 in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup

 are bent shape molecule.
are bent shape molecule.