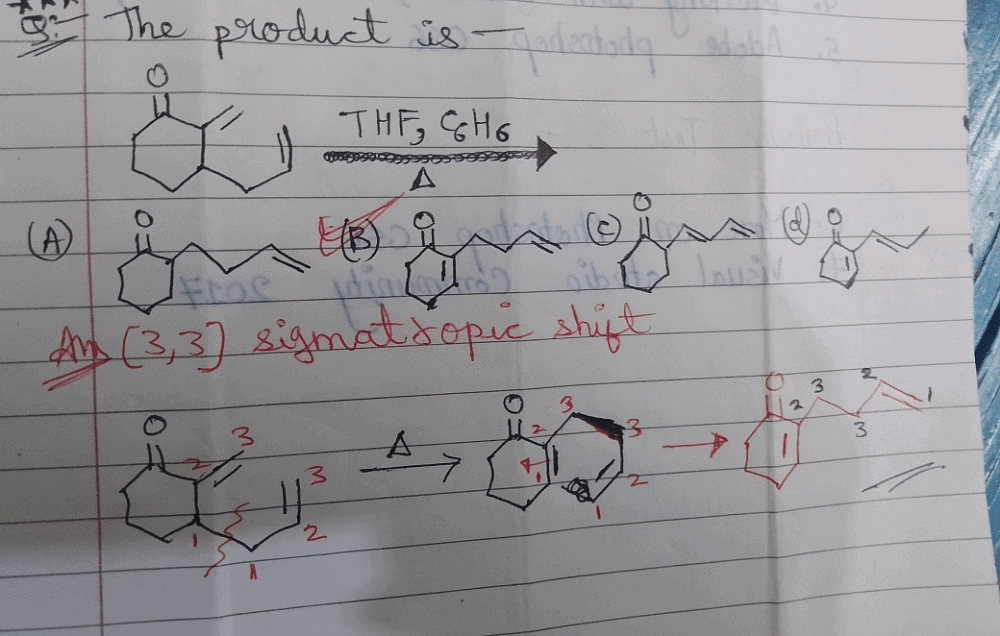All Exams >
Chemistry >
Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry >
All Questions
All questions of Aromatic and Heterocyclic Chemistry for Chemistry Exam
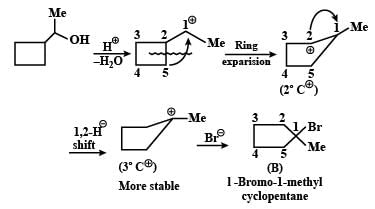
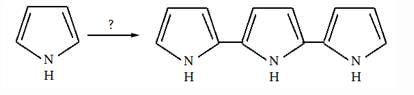
Identify the major Product P in the following two–step reaction: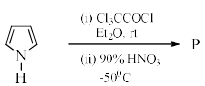
- a)
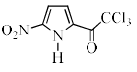
- b)
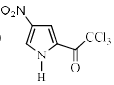
- c)
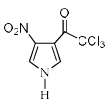
- d)
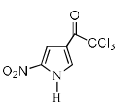
Correct answer is option 'A'. Can you explain this answer?
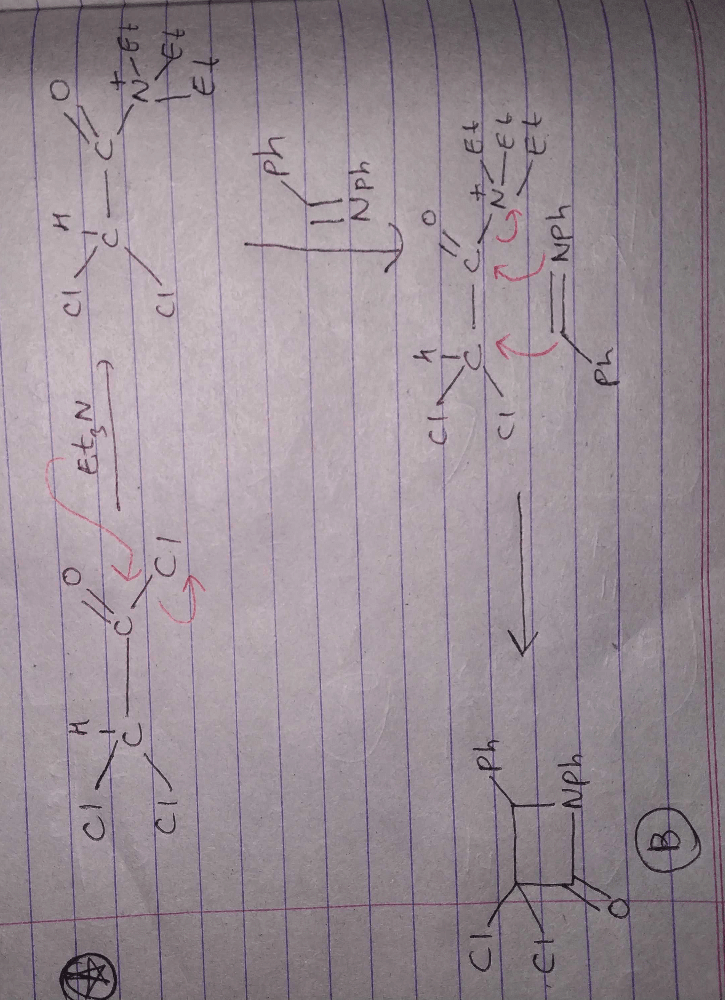
Identify the major Product P in the following two–step reaction:
a)
b)
c)
d)
|
|
Ajit Yadav answered |
Firstly pyrrole undergoes electrophilic substitution reaction preferentially at c 2 then resulting give meta directing and then nitration give product b
Diels-Alder reaction normally yields endo-adduct as a major product. This is due to:- a) Higher stability of the product.
- b) Faster rate of formation of the endo product.
- c) Steric hindrance.
- d) Secondary orbital interactions between a diene and a dienophile.
Correct answer is option 'D'. Can you explain this answer?
Diels-Alder reaction normally yields endo-adduct as a major product. This is due to:
a)
Higher stability of the product.
b)
Faster rate of formation of the endo product.
c)
Steric hindrance.
d)
Secondary orbital interactions between a diene and a dienophile.

|
Saranya Mehta answered |
Introduction:
The Diels-Alder reaction is a powerful synthetic tool used to construct cyclic compounds. It involves the reaction between a conjugated diene and a dienophile to form a cyclic product known as the cycloadduct. In most cases, the major product obtained in the Diels-Alder reaction is the endo-adduct. This preference for the endo product can be explained by several factors.
Explanation:
1. Secondary orbital interactions:
The endo-adduct is favored due to the presence of secondary orbital interactions between the diene and dienophile. These interactions occur between the non-bonding electron pair on the diene and the empty orbital on the dienophile. This stabilizes the transition state leading to the formation of the endo product. The interaction is more favorable in the endo position compared to the exo position, leading to the preference for the endo-adduct.
2. Steric hindrance:
Steric hindrance also plays a role in determining the regioselectivity of the Diels-Alder reaction. The endo-product is often favored due to the lower steric hindrance in the transition state leading to its formation. The exo-product is hindered by the presence of bulky substituents that can lead to steric clashes, making the formation of the endo-product more favorable.
3. Stability of the product:
The endo-adduct is generally more stable than the exo-adduct due to the spatial arrangement of substituents. The endo-product often has substituents arranged in a more favorable conformation, leading to lower steric strain and increased stability. This stability contributes to the preference for the endo product in the Diels-Alder reaction.
4. Faster rate of formation:
In some cases, the endo-product may also be favored due to the faster rate of formation. The transition state leading to the endo-product may have a lower activation energy compared to the transition state leading to the exo-product. This kinetic preference can be attributed to a combination of factors, including orbital interactions and steric effects.
Conclusion:
In conclusion, the preference for the endo-adduct in the Diels-Alder reaction can be attributed to secondary orbital interactions, steric hindrance, stability of the product, and sometimes a faster rate of formation. These factors work together to determine the regioselectivity of the reaction, leading to the predominant formation of the endo product.
The Diels-Alder reaction is a powerful synthetic tool used to construct cyclic compounds. It involves the reaction between a conjugated diene and a dienophile to form a cyclic product known as the cycloadduct. In most cases, the major product obtained in the Diels-Alder reaction is the endo-adduct. This preference for the endo product can be explained by several factors.
Explanation:
1. Secondary orbital interactions:
The endo-adduct is favored due to the presence of secondary orbital interactions between the diene and dienophile. These interactions occur between the non-bonding electron pair on the diene and the empty orbital on the dienophile. This stabilizes the transition state leading to the formation of the endo product. The interaction is more favorable in the endo position compared to the exo position, leading to the preference for the endo-adduct.
2. Steric hindrance:
Steric hindrance also plays a role in determining the regioselectivity of the Diels-Alder reaction. The endo-product is often favored due to the lower steric hindrance in the transition state leading to its formation. The exo-product is hindered by the presence of bulky substituents that can lead to steric clashes, making the formation of the endo-product more favorable.
3. Stability of the product:
The endo-adduct is generally more stable than the exo-adduct due to the spatial arrangement of substituents. The endo-product often has substituents arranged in a more favorable conformation, leading to lower steric strain and increased stability. This stability contributes to the preference for the endo product in the Diels-Alder reaction.
4. Faster rate of formation:
In some cases, the endo-product may also be favored due to the faster rate of formation. The transition state leading to the endo-product may have a lower activation energy compared to the transition state leading to the exo-product. This kinetic preference can be attributed to a combination of factors, including orbital interactions and steric effects.
Conclusion:
In conclusion, the preference for the endo-adduct in the Diels-Alder reaction can be attributed to secondary orbital interactions, steric hindrance, stability of the product, and sometimes a faster rate of formation. These factors work together to determine the regioselectivity of the reaction, leading to the predominant formation of the endo product.
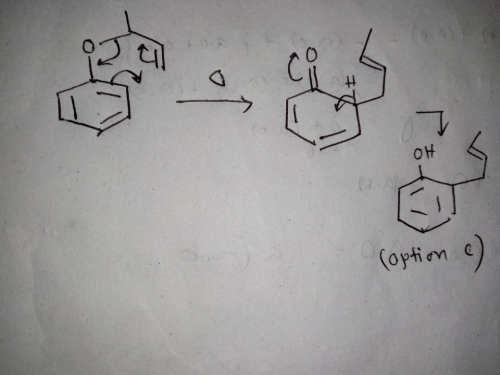
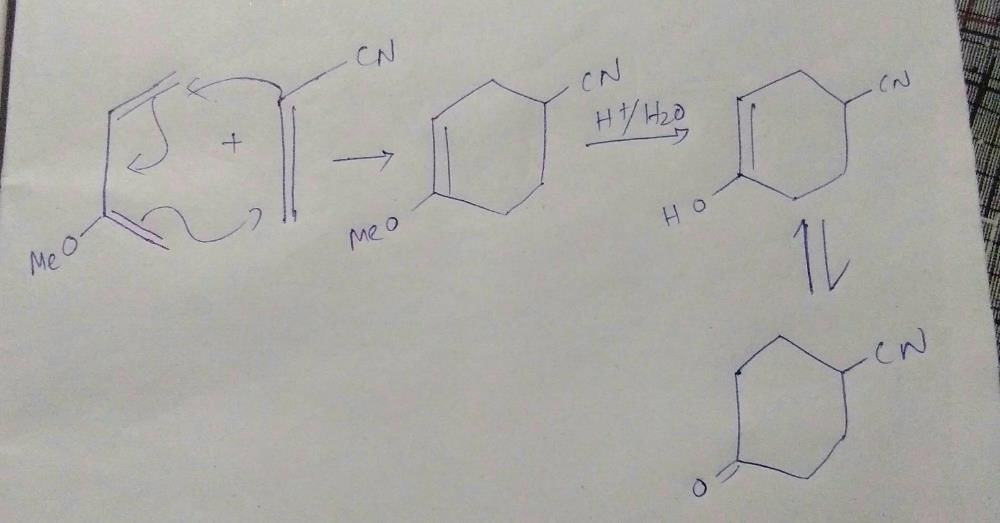
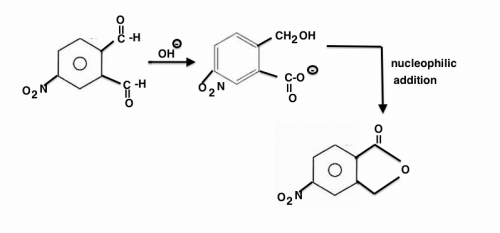
The major product of the following is:
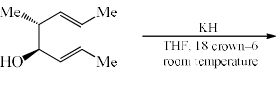
- a)
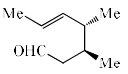
- b)
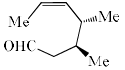
- c)
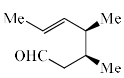
- d)
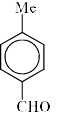
Correct answer is option 'A'. Can you explain this answer?
The major product of the following is:
a)
b)
c)
d)
|
|
Vedika Singh answered |
Six membered transition state of chair form will give option A.
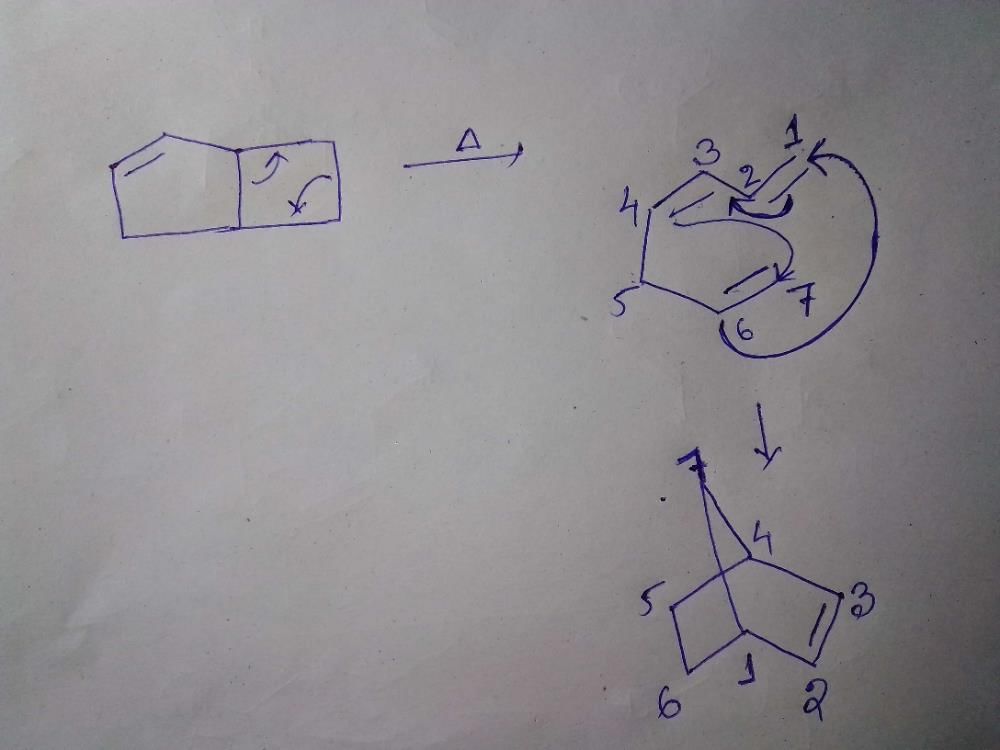
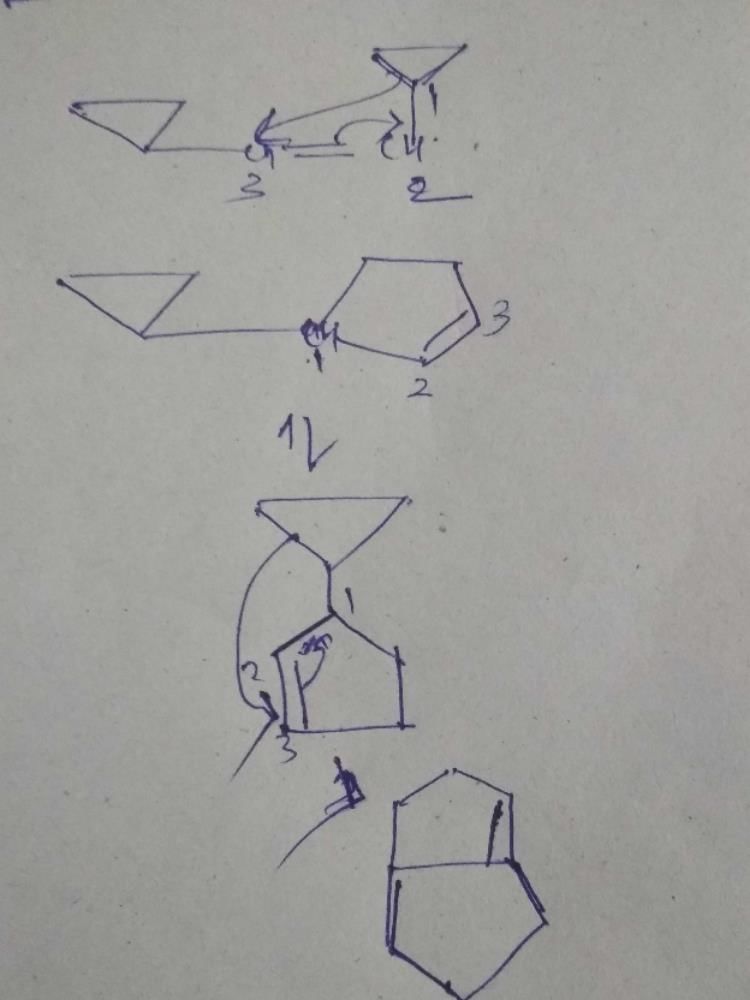
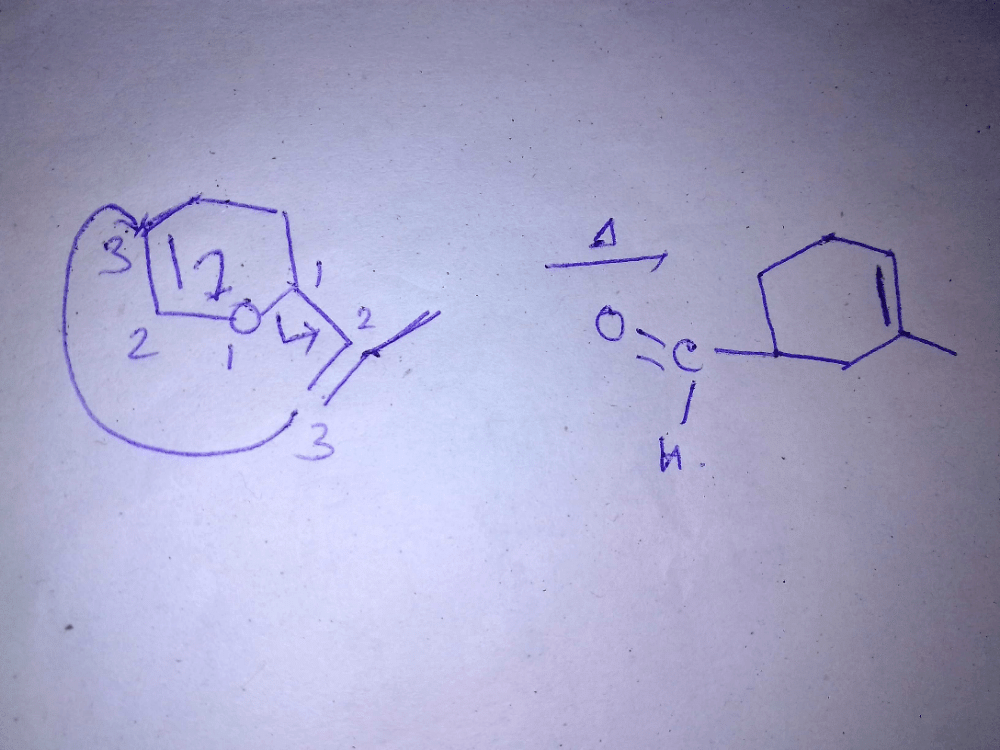
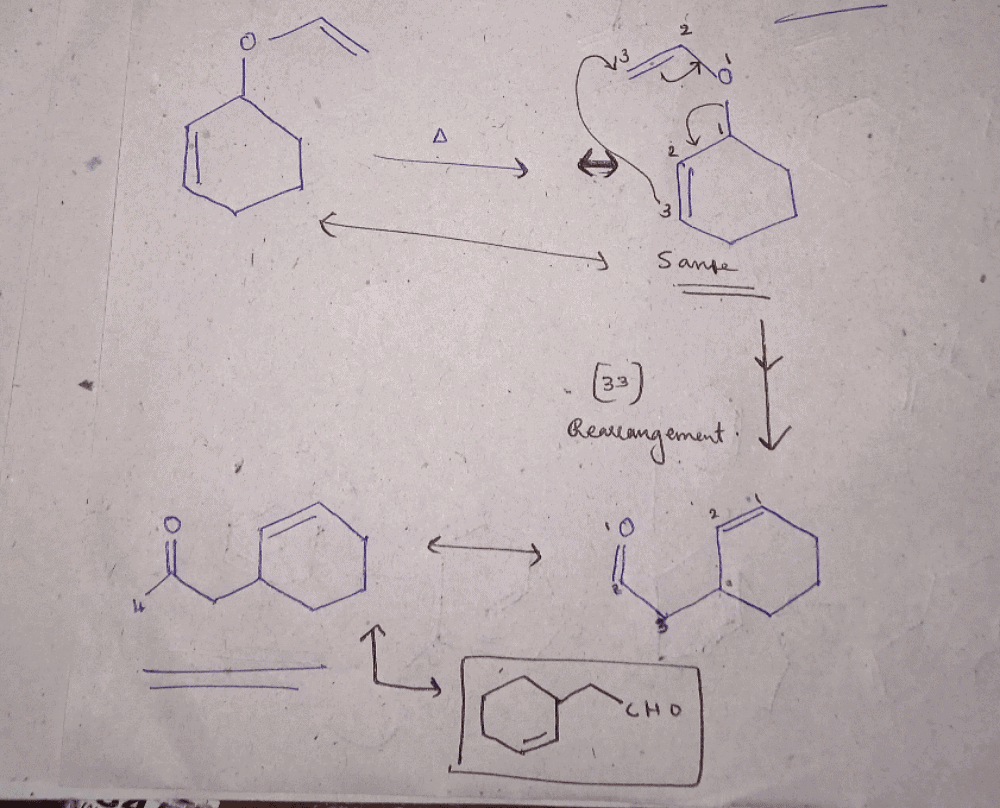
Consider the following statements:(I) Clasisen rearrangement is a [3, 3] sigmatropic rearrangement.
(II) Cope rearrangement proceeds via is chair like transition state.
(III) In the photochemical [2 + 2] cycloaddition of ethylene overlapping of HOMO of one molecule with LUMO of the other molecular takes place.Select the correct statements.- a)I and III
- b)II and III
- c)Only II
- d)I and II
Correct answer is option 'D'. Can you explain this answer?
Consider the following statements:
(I) Clasisen rearrangement is a [3, 3] sigmatropic rearrangement.
(II) Cope rearrangement proceeds via is chair like transition state.
(III) In the photochemical [2 + 2] cycloaddition of ethylene overlapping of HOMO of one molecule with LUMO of the other molecular takes place.
(II) Cope rearrangement proceeds via is chair like transition state.
(III) In the photochemical [2 + 2] cycloaddition of ethylene overlapping of HOMO of one molecule with LUMO of the other molecular takes place.
Select the correct statements.
a)
I and III
b)
II and III
c)
Only II
d)
I and II

|
Bijoy Patel answered |
Explanation:
Therefore, the correct option is 'D' - I and II.
- Claisen rearrangement: It is a [3, 3] sigmatropic rearrangement, which involves the migration of an allyl group from one oxygen atom to another oxygen atom, with the formation of a new carbon-carbon bond. Hence, statement I is correct.
- Cope rearrangement: It is a [3, 3] sigmatropic rearrangement, which involves the migration of a hydrocarbon group from one carbon atom to an adjacent carbon atom, with the formation of a new carbon-carbon bond. This reaction proceeds via a chair-like transition state. Hence, statement II is correct.
- Photochemical [2+2] cycloaddition: This reaction involves the formation of a cyclobutane ring from two molecules of ethylene under the influence of light. In this reaction, the highest occupied molecular orbital (HOMO) of one molecule overlaps with the lowest unoccupied molecular orbital (LUMO) of the other molecule. Hence, statement III is also correct.
Therefore, the correct option is 'D' - I and II.
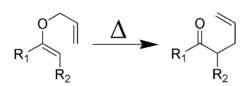
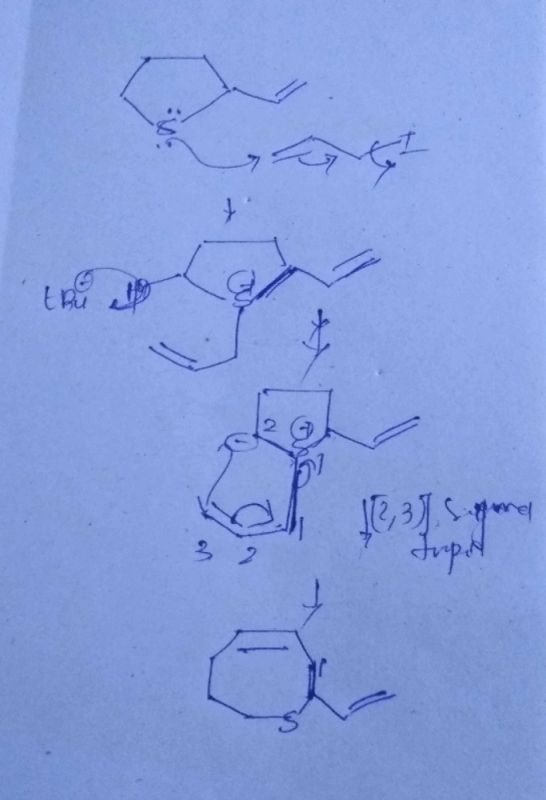
Claisen rearrangement is an example of:- a)[2, 3] sigmatropic rearrangement.
- b)[2, 4] sigmatropic rearrangement.
- c)[1, 5] sigmatropic rearrangement.
- d)[3, 3] sigmatropic rearrangement.
Correct answer is option 'D'. Can you explain this answer?
Claisen rearrangement is an example of:
a)
[2, 3] sigmatropic rearrangement.
b)
[2, 4] sigmatropic rearrangement.
c)
[1, 5] sigmatropic rearrangement.
d)
[3, 3] sigmatropic rearrangement.

|
Ameya Reddy answered |
Claisen rearrangement is an example of [3,3] sigmatropic rearrangement.
Explanation:
- Rearrangement reactions involve the movement of atoms or groups within a molecule, resulting in a new structure.
- Sigmatropic rearrangement is a type of rearrangement reaction that involves the simultaneous breaking and forming of sigma bonds.
- In Claisen rearrangement, a vinyl ether undergoes rearrangement to form an allyl ether, with migration of a carbon-carbon double bond from one position to another within the molecule.
- The reaction involves the simultaneous breaking and forming of three sigma bonds, hence it is a [3,3] sigmatropic rearrangement.
- Other examples of sigmatropic rearrangements include [2,3], [3,3], [1,3], [1,5], [2,5], etc. depending on the number of atoms involved in the rearrangement and the direction of migration.
In summary, Claisen rearrangement is an example of [3,3] sigmatropic rearrangement, which involves the simultaneous breaking and forming of three sigma bonds.
Explanation:
- Rearrangement reactions involve the movement of atoms or groups within a molecule, resulting in a new structure.
- Sigmatropic rearrangement is a type of rearrangement reaction that involves the simultaneous breaking and forming of sigma bonds.
- In Claisen rearrangement, a vinyl ether undergoes rearrangement to form an allyl ether, with migration of a carbon-carbon double bond from one position to another within the molecule.
- The reaction involves the simultaneous breaking and forming of three sigma bonds, hence it is a [3,3] sigmatropic rearrangement.
- Other examples of sigmatropic rearrangements include [2,3], [3,3], [1,3], [1,5], [2,5], etc. depending on the number of atoms involved in the rearrangement and the direction of migration.
In summary, Claisen rearrangement is an example of [3,3] sigmatropic rearrangement, which involves the simultaneous breaking and forming of three sigma bonds.
Hydrogen bonding is maximum in:
- a)Ethanol
- b)Ethyl chloride
- c)Diethyl ether
- d)Triethylamine
Correct answer is option 'A'. Can you explain this answer?
Hydrogen bonding is maximum in:
a)
Ethanol
b)
Ethyl chloride
c)
Diethyl ether
d)
Triethylamine

|
Shail Ghoshal answered |
Hydrogen bonding is maximum in ethanol.
Explanation:
What is hydrogen bonding?
Hydrogen bonding is a special type of intermolecular force that occurs between molecules containing hydrogen atoms bonded to highly electronegative elements such as oxygen, nitrogen, or fluorine. It is a strong dipole-dipole interaction that results in the formation of a partially positive hydrogen atom and a partially negative atom.
Factors affecting hydrogen bonding:
There are several factors that influence the strength of hydrogen bonding, including:
1. Electronegativity difference: The greater the electronegativity difference between hydrogen and the atom it is bonded to, the stronger the hydrogen bonding.
2. Size of the atom: Smaller atoms with higher electronegativity can form stronger hydrogen bonds.
3. Number of hydrogen bonding sites: The more hydrogen atoms bonded to electronegative atoms, the more hydrogen bonding sites are available.
4. Molecular shape: The presence of hydrogen bonding sites in a linear or planar arrangement increases the strength of hydrogen bonding.
Comparison of the given compounds:
a) Ethanol: Ethanol (CH3CH2OH) contains an -OH group, which can form hydrogen bonds. The electronegative oxygen atom attracts the hydrogen atom, resulting in the formation of strong hydrogen bonds between ethanol molecules.
b) Ethyl chloride: Ethyl chloride (CH3CH2Cl) does not contain any hydrogen bonding sites. The chlorine atom is not electronegative enough to form strong hydrogen bonds.
c) Diethyl ether: Diethyl ether (CH3CH2OCH2CH3) contains an oxygen atom, but it does not have any hydrogen bonding sites. The oxygen atom is not bonded to a hydrogen atom.
d) Triethylamine: Triethylamine (N(C2H5)3) does not contain any hydrogen bonding sites. Although it contains a nitrogen atom, it is not bonded to a hydrogen atom.
Conclusion:
Among the given compounds, ethanol has the maximum hydrogen bonding because it contains an -OH group, which can form strong hydrogen bonds. The other compounds do not have hydrogen bonding sites or have less electronegative atoms, resulting in weaker or no hydrogen bonding.
Explanation:
What is hydrogen bonding?
Hydrogen bonding is a special type of intermolecular force that occurs between molecules containing hydrogen atoms bonded to highly electronegative elements such as oxygen, nitrogen, or fluorine. It is a strong dipole-dipole interaction that results in the formation of a partially positive hydrogen atom and a partially negative atom.
Factors affecting hydrogen bonding:
There are several factors that influence the strength of hydrogen bonding, including:
1. Electronegativity difference: The greater the electronegativity difference between hydrogen and the atom it is bonded to, the stronger the hydrogen bonding.
2. Size of the atom: Smaller atoms with higher electronegativity can form stronger hydrogen bonds.
3. Number of hydrogen bonding sites: The more hydrogen atoms bonded to electronegative atoms, the more hydrogen bonding sites are available.
4. Molecular shape: The presence of hydrogen bonding sites in a linear or planar arrangement increases the strength of hydrogen bonding.
Comparison of the given compounds:
a) Ethanol: Ethanol (CH3CH2OH) contains an -OH group, which can form hydrogen bonds. The electronegative oxygen atom attracts the hydrogen atom, resulting in the formation of strong hydrogen bonds between ethanol molecules.
b) Ethyl chloride: Ethyl chloride (CH3CH2Cl) does not contain any hydrogen bonding sites. The chlorine atom is not electronegative enough to form strong hydrogen bonds.
c) Diethyl ether: Diethyl ether (CH3CH2OCH2CH3) contains an oxygen atom, but it does not have any hydrogen bonding sites. The oxygen atom is not bonded to a hydrogen atom.
d) Triethylamine: Triethylamine (N(C2H5)3) does not contain any hydrogen bonding sites. Although it contains a nitrogen atom, it is not bonded to a hydrogen atom.
Conclusion:
Among the given compounds, ethanol has the maximum hydrogen bonding because it contains an -OH group, which can form strong hydrogen bonds. The other compounds do not have hydrogen bonding sites or have less electronegative atoms, resulting in weaker or no hydrogen bonding.
Chapter doubts & questions for Aromatic and Heterocyclic Chemistry - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Aromatic and Heterocyclic Chemistry - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev