All Exams >
Grade 9 >
Chemistry for Grade 9 >
All Questions
All questions of Is Matter Around Us Pure? for Grade 9 Exam
The formation of water from oxygen and hydrogen is a _______ .- a)Physical change
- b)Chemical change
- c)Reversible change
- d)Both Physical and Reversible change
Correct answer is option 'B'. Can you explain this answer?
The formation of water from oxygen and hydrogen is a _______ .
a)
Physical change
b)
Chemical change
c)
Reversible change
d)
Both Physical and Reversible change
|
|
Jaideep Sharma answered |
Chemical Change:
When hydrogen and oxygen combine to form water, a new substance with different properties is formed. This is a chemical change.
Explanation:
Chemical change is a process in which one or more substances are changed into new substances with different physical and chemical properties. In the formation of water from hydrogen and oxygen, the hydrogen and oxygen molecules bond together to form a new molecule of water. This reaction is accompanied by the release of energy in the form of heat and light.
The chemical equation for the reaction is:
2H2 + O2 → 2H2O
Here, two molecules of hydrogen (H2) and one molecule of oxygen (O2) combine to form two molecules of water (H2O).
Reversible Change:
The formation of water from hydrogen and oxygen is not a reversible change. Once the reaction takes place, it is not possible to separate the water molecules back into hydrogen and oxygen.
Conclusion:
Hence, the correct option is B. Chemical change.
When hydrogen and oxygen combine to form water, a new substance with different properties is formed. This is a chemical change.
Explanation:
Chemical change is a process in which one or more substances are changed into new substances with different physical and chemical properties. In the formation of water from hydrogen and oxygen, the hydrogen and oxygen molecules bond together to form a new molecule of water. This reaction is accompanied by the release of energy in the form of heat and light.
The chemical equation for the reaction is:
2H2 + O2 → 2H2O
Here, two molecules of hydrogen (H2) and one molecule of oxygen (O2) combine to form two molecules of water (H2O).
Reversible Change:
The formation of water from hydrogen and oxygen is not a reversible change. Once the reaction takes place, it is not possible to separate the water molecules back into hydrogen and oxygen.
Conclusion:
Hence, the correct option is B. Chemical change.
Which of the following is not a mixture? a) Soap solutionb) Bloodc) Carbon dioxided) CoalCorrect answer is option 'C'. Can you explain this answer?
|
|
Gaurav Kumar answered |
Carbon dioxide is not a mixture since it contain fixed ratio of oxygen and carbon in 2:1, which is a property of compound not a mixture.
Which of the following is not a pure substance?- a)Mercury
- b)Distilled water
- c)Nitric acid
- d)Tap water
Correct answer is 'D'. Can you explain this answer?
Which of the following is not a pure substance?
a)
Mercury
b)
Distilled water
c)
Nitric acid
d)
Tap water

|
Varun Kumar answered |
Tap water is not a pure substance because it is mixed with chemicals that purify it and if it was from the ground it has naturally occurring minerals mixed in it.
A desert is ...................- a)an endless stretch of sand
- b)without rainfall
- c)without vegetation
- d)all of the above
Correct answer is option 'D'. Can you explain this answer?
A desert is ...................
a)
an endless stretch of sand
b)
without rainfall
c)
without vegetation
d)
all of the above
|
|
Parth Nambiar answered |
Desert
Desert is a geographical region that is characterized by extreme dryness and lack of precipitation. It is a vast expanse of land that receives very little rainfall, and as a result, vegetation is scarce, and sand is abundant.
Endless stretch of sand
Deserts are often associated with endless stretches of sand dunes, but this is not always the case. While sand dunes are a common feature of many deserts, there are also rocky deserts, and deserts that are characterized by vast plains of gravel and dirt.
Without rainfall
Deserts are defined by their lack of rainfall, with many receiving less than 10 inches of rain per year. This lack of rainfall is often due to their location, such as being situated in areas that are far from oceans or other moisture sources.
Without vegetation
The extreme dryness of deserts means that vegetation is scarce, with many deserts being home to only a few types of plants that are adapted to the harsh desert environment. These plants often have specialized adaptations to help them survive in the arid conditions, such as the ability to store water in their leaves or roots.
All of the above
In conclusion, deserts are characterized by their lack of rainfall, scarcity of vegetation, and often vast stretches of sand or rocky terrain. These features make deserts some of the most inhospitable places on Earth, but they are also home to a variety of unique and fascinating plant and animal species that have adapted to the harsh desert environment.
Desert is a geographical region that is characterized by extreme dryness and lack of precipitation. It is a vast expanse of land that receives very little rainfall, and as a result, vegetation is scarce, and sand is abundant.
Endless stretch of sand
Deserts are often associated with endless stretches of sand dunes, but this is not always the case. While sand dunes are a common feature of many deserts, there are also rocky deserts, and deserts that are characterized by vast plains of gravel and dirt.
Without rainfall
Deserts are defined by their lack of rainfall, with many receiving less than 10 inches of rain per year. This lack of rainfall is often due to their location, such as being situated in areas that are far from oceans or other moisture sources.
Without vegetation
The extreme dryness of deserts means that vegetation is scarce, with many deserts being home to only a few types of plants that are adapted to the harsh desert environment. These plants often have specialized adaptations to help them survive in the arid conditions, such as the ability to store water in their leaves or roots.
All of the above
In conclusion, deserts are characterized by their lack of rainfall, scarcity of vegetation, and often vast stretches of sand or rocky terrain. These features make deserts some of the most inhospitable places on Earth, but they are also home to a variety of unique and fascinating plant and animal species that have adapted to the harsh desert environment.
What type of mixture is obtained on continuous stirring when we add one spoon of sugar to water?- a)Homogeneous mixture
- b)Colloid
- c)Suspension
- d)Heterogeneous mixture
Correct answer is option 'A'. Can you explain this answer?
What type of mixture is obtained on continuous stirring when we add one spoon of sugar to water?
a)
Homogeneous mixture
b)
Colloid
c)
Suspension
d)
Heterogeneous mixture
|
|
Anita Menon answered |
The homogeneous mixture is obtained because the particles of sugar are so small that it can be diffused to the intermolecular space. The composition remains is the same throughout.
Which of the following colloid is a gel?- a)Fog
- b)Cheese
- c)Milk
- d)Smoke
Correct answer is option 'B'. Can you explain this answer?
Which of the following colloid is a gel?
a)
Fog
b)
Cheese
c)
Milk
d)
Smoke
|
|
Vivek Rana answered |
The colloidal system constituting the liquid as the dispersed phase and the solid as the dispersion medium is known as gel. There are some sols that have a high concentration of dispersed solid and change spontaneously into semi solid form on cooling. These are known as gels and the process is known as gelatin
The smell of hydrogen sulphide (H2S) gas- a)pleasant
- b)of rotten egg
- c)of burning sulphur
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
The smell of hydrogen sulphide (H2S) gas
a)
pleasant
b)
of rotten egg
c)
of burning sulphur
d)
None of these
|
|
Pooja Shah answered |
Hydrogen sulfide is a flammable, colorless gas with a characteristic odour of rotten eggs. It is commonly known as hydrosulfuric acid, sewer gas, and stink damp. People can smell it at low levels.
Which of the following involves physical change?- a)Grinding
- b)Tearing
- c)Cutting
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
Which of the following involves physical change?
a)
Grinding
b)
Tearing
c)
Cutting
d)
All of the above

|
Aryan answered |
Grinding Clearing and cutting all come under physical change..........................
If a solution contains 60g of common salt in 340g of water, the mass by mass percentage will be:- a)25 %
- b)15 %
- c)20 %
- d)17.6 %
Correct answer is 'B'. Can you explain this answer?
If a solution contains 60g of common salt in 340g of water, the mass by mass percentage will be:
a)
25 %
b)
15 %
c)
20 %
d)
17.6 %
|
|
Vivek Rana answered |
Concentration of solution is mass of solute upon mass of solution by 100 so mass of solute is 60g and mass of solvent is 340 so mass of solution is 340+60 is equal to 400 we put these values in formula so 60/400×100=15%.
A colloid with a solid dispersed phase and liquid dispersing medium is called:- a)Foam
- b)Gel
- c)Sol
- d)Emulsion
Correct answer is option 'C'. Can you explain this answer?
A colloid with a solid dispersed phase and liquid dispersing medium is called:
a)
Foam
b)
Gel
c)
Sol
d)
Emulsion

|
Vivek Kumar answered |
Because sol is a colloids in which tiny solid particles are dispersed in a liquid medium.thats why correct answer is sol
Name the solvent which is known as universal solvent.- a)Ethanol
- b)Benzene
- c)Vinegar
- d)Water
Correct answer is option 'D'. Can you explain this answer?
Name the solvent which is known as universal solvent.
a)
Ethanol
b)
Benzene
c)
Vinegar
d)
Water
|
|
Anita Menon answered |
Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. And, water is called the "universal solvent" because it dissolves more substances than any other liquid. This is important to every living thing on earth. It means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients.
It is water's chemical composition and physical attributes that make it such an excellent solvent. Water molecules have a polar arrangement of the oxygen and hydrogen atoms—one side (hydrogen) has a positive electrical charge and the other side (oxygen) had a negative charge. This allows the water molecule to become attracted to many other different types of molecules. Water can become so heavily attracted to a different molecule, like salt (NaCl), that it can disrupt the attractive forces that hold the sodium and chloride in the salt molecule together and, thus, dissolve it.
100 mL solution contains 3.5 g salt. The mass by volume percentage (m/v) of the solution is- a)3.5 %
- b)2.5 %
- c)35 %
- d)25 %
Correct answer is option 'A'. Can you explain this answer?
100 mL solution contains 3.5 g salt. The mass by volume percentage (m/v) of the solution is
a)
3.5 %
b)
2.5 %
c)
35 %
d)
25 %
|
|
Amit Sharma answered |
FORMULA FOR MASS BY VOLUME PERCENTAGE IS = TOTAL MASS OF SOLUTE / TOTAL VOLUME × 100 MASS OF SOLUTE = 3.5 G VOLUME = 100 THERE FOR 3.5G/100 ×100= 3.5 %
Most paints are:- a)Gels
- b)Suspensions
- c)Emulsions
- d)Sols
Correct answer is option 'D'. Can you explain this answer?
Most paints are:
a)
Gels
b)
Suspensions
c)
Emulsions
d)
Sols
|
|
Naina Sharma answered |
Most paints are sols in which tiny solid particles are dispersed in a liquid medium.
An example of a colloid is:- a)Sugar solution
- b)Milk
- c)Oxygen
- d)Water
Correct answer is option 'B'. Can you explain this answer?
An example of a colloid is:
a)
Sugar solution
b)
Milk
c)
Oxygen
d)
Water
|
|
Anita Menon answered |
Types of colloids. Colloids are common in everyday life. Some examples include whipped cream, mayonnaise, milk, butter, gelatin, jelly, muddy water, plaster, colored glass, and paper. Every colloid consists of two parts: colloidal particles and the dispersing medium.
The process of separation of insoluble solids from a liquid is called:- a)Filtration
- b)Decantation
- c)Crystallisation
- d)Evaporation
Correct answer is option 'A'. Can you explain this answer?
The process of separation of insoluble solids from a liquid is called:
a)
Filtration
b)
Decantation
c)
Crystallisation
d)
Evaporation
|
|
Krishna Iyer answered |
Filtration is a method for separating an insoluble solid from a liquid. When a mixture of sand and water is filtered: the sand stays behind in the filter paper (it becomes the residue ) the water passes through the filter paper (it becomes the filtrate).
Which of the following statement is true for colloids?- a)Colloid is a homogeneous mixture.
- b)Particles of a colloid can be seen by naked eye.
- c)Particles of colloid scatter a beam of light passing through it.
- d)All of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following statement is true for colloids?
a)
Colloid is a homogeneous mixture.
b)
Particles of a colloid can be seen by naked eye.
c)
Particles of colloid scatter a beam of light passing through it.
d)
All of these
|
|
Vivek Rana answered |
A starch solution is a colloidal solution. in a colloidal solution, particles are relatively big. so, when a beam of light is passed, the path of light is visible. it scatters. this scattering of beam of light through a colloidal solution is called tyndall effect.
Which of the following statements is incorrect about physical changes?- a)There is no gain or loss of energy.
- b)It is permanent and irreversible.
- c)Composition of the substance remains same.
- d)No new substance is formed.
Correct answer is option 'B'. Can you explain this answer?
Which of the following statements is incorrect about physical changes?
a)
There is no gain or loss of energy.
b)
It is permanent and irreversible.
c)
Composition of the substance remains same.
d)
No new substance is formed.
|
|
Gopal Majumdar answered |
Explanation:
Physical changes are changes in the physical properties of a substance, such as shape, size, state, or appearance. They do not involve any change in the chemical composition of the substance. Some common examples of physical changes are melting, freezing, boiling, condensation, sublimation, and dissolution.
Now, let us analyze the given statements one by one:
a) There is no gain or loss of energy: This statement is correct. Physical changes do not involve any change in the energy of the substance. The energy may be required to carry out the change, but it is not gained or lost by the substance itself.
b) It is permanent and irreversible: This statement is incorrect. Physical changes are usually temporary and reversible. For example, if we melt an ice cube, it will turn into water, but we can freeze the water again to get back the ice cube. Similarly, if we dissolve salt in water, we can evaporate the water to get back the salt.
c) Composition of the substance remains the same: This statement is correct. Physical changes do not involve any change in the chemical composition of the substance. The atoms or molecules of the substance remain the same before and after the change.
d) No new substance is formed: This statement is correct. Physical changes do not involve any chemical reaction, so no new substance is formed. The substance may change its state or appearance, but it remains the same substance.
Therefore, the correct answer is option 'B' which says that physical changes are permanent and irreversible. In reality, physical changes are usually temporary and reversible.
Physical changes are changes in the physical properties of a substance, such as shape, size, state, or appearance. They do not involve any change in the chemical composition of the substance. Some common examples of physical changes are melting, freezing, boiling, condensation, sublimation, and dissolution.
Now, let us analyze the given statements one by one:
a) There is no gain or loss of energy: This statement is correct. Physical changes do not involve any change in the energy of the substance. The energy may be required to carry out the change, but it is not gained or lost by the substance itself.
b) It is permanent and irreversible: This statement is incorrect. Physical changes are usually temporary and reversible. For example, if we melt an ice cube, it will turn into water, but we can freeze the water again to get back the ice cube. Similarly, if we dissolve salt in water, we can evaporate the water to get back the salt.
c) Composition of the substance remains the same: This statement is correct. Physical changes do not involve any change in the chemical composition of the substance. The atoms or molecules of the substance remain the same before and after the change.
d) No new substance is formed: This statement is correct. Physical changes do not involve any chemical reaction, so no new substance is formed. The substance may change its state or appearance, but it remains the same substance.
Therefore, the correct answer is option 'B' which says that physical changes are permanent and irreversible. In reality, physical changes are usually temporary and reversible.
What do you understand by the term concentrated solution?- a)Solution containing no solute
- b)Solution with low solute concentration
- c)Solution in which no more solute can be dissolved
- d)Solution with high solute concentration
Correct answer is option 'D'. Can you explain this answer?
What do you understand by the term concentrated solution?
a)
Solution containing no solute
b)
Solution with low solute concentration
c)
Solution in which no more solute can be dissolved
d)
Solution with high solute concentration
|
|
Sarita Reddy answered |
A concentrated solution contains a relatively large amount of the solute in the same volume of solvent. Most commercial acids are concentrated solutions. For example, commercial hydrochloric acid (HCl) and sulfuric acid (H2SO4) are concentrated solutions.
A dilute solution is one that contains a relatively small amount of the solute in a given volume of solvent. Tap water is an example of a dilute
solution; it contains very small quantities of dissolved minerals.
Which of the following is not true for a mixture?- a)A mixture can be separated into its constituents.
- b)Energy is not given out in the preparation of a mixture.
- c)A mixture has a fixed melting point.
- d)The composition of a mixture is variable
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not true for a mixture?
a)
A mixture can be separated into its constituents.
b)
Energy is not given out in the preparation of a mixture.
c)
A mixture has a fixed melting point.
d)
The composition of a mixture is variable
|
|
Jyoti Kapoor answered |
A mixture does not have a fixed melting point, boiling point etc. On the other hand, compounds have a a fixed melting point, boiling point.
40 g of common salt is dissolved in 320 g of water. The mass percentage of salt is - a)11.1%
- b)12.5%
- c)15%
- d)10%
Correct answer is option 'A'. Can you explain this answer?
40 g of common salt is dissolved in 320 g of water. The mass percentage of salt is
a)
11.1%
b)
12.5%
c)
15%
d)
10%
|
|
Anita Menon answered |
Mass of common salt (solute) = 40g
Mass of water (soluted) = 320g
Mass of solution = 320 + 40 = 360g
Concentration of solution = M(mass of solute) / mass of solution = 40/360 x 100 = 11.11%
Mass of water (soluted) = 320g
Mass of solution = 320 + 40 = 360g
Concentration of solution = M(mass of solute) / mass of solution = 40/360 x 100 = 11.11%
Which of the following statement is correct?- a)Both burning of a paper and cooking of food are examples of a physical change
- b)Both burning of wood and cooking of food are are examples of a chemical change.
- c)Burning of a paper is a physical change and cooking of food is a chemical change.
- d)Burning of a paper is chemical change and cooking of food is a physical change.
Correct answer is option 'B'. Can you explain this answer?
Which of the following statement is correct?
a)
Both burning of a paper and cooking of food are examples of a physical change
b)
Both burning of wood and cooking of food are are examples of a chemical change.
c)
Burning of a paper is a physical change and cooking of food is a chemical change.
d)
Burning of a paper is chemical change and cooking of food is a physical change.
|
|
Avinash Patel answered |
Examples of Chemical Changes
A new compound (product) results from a chemical change as the atoms rearrange themselves to form new chemical bonds.
A new compound (product) results from a chemical change as the atoms rearrange themselves to form new chemical bonds.
- Burning wood
- Souring milk
- Mixing acid and base
- Digesting food
- Cooking an egg
- Heating sugar to form caramel
- Baking a cake
- Rusting of iron
Which of the following statement is not true?- a)The solubility of gases in liquids decreases on increasing the temperature.
- b)The solubility of solids in liquids remain unaffected by changes in pressure.
- c)The solubility of solids in liquids increases on increasing the temperature.
- d)The solubility of gases in liquids increases on increasing the temperature.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is not true?
a)
The solubility of gases in liquids decreases on increasing the temperature.
b)
The solubility of solids in liquids remain unaffected by changes in pressure.
c)
The solubility of solids in liquids increases on increasing the temperature.
d)
The solubility of gases in liquids increases on increasing the temperature.
|
|
Jyoti Kapoor answered |
The reason for this gas solubility relationship with temperature is very similar to the reason that vapor pressure increases with temperature. Increased temperature causes an increase in kinetic energy. The higher kinetic energy causes more motion in molecules which break intermolecular bonds and escape from solution. As the temperature increases, the solubility of a gas decreases.
Match the following with correct response. 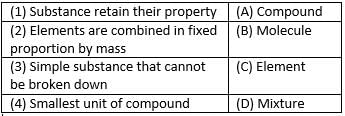
- a)1-C, 2-B, 3-D, 4-A
- b)1-A, 2-C, 3-B, 4-D
- c)1-D, 2-A, 3-C, 4-B
- d)1-B, 2-D, 3-A, 4-C
Correct answer is option 'C'. Can you explain this answer?
Match the following with correct response.

a)
1-C, 2-B, 3-D, 4-A
b)
1-A, 2-C, 3-B, 4-D
c)
1-D, 2-A, 3-C, 4-B
d)
1-B, 2-D, 3-A, 4-C
|
|
Pooja Shah answered |
Mixtures are one product of mechanically blending or mixing chemical substances such as elements and compounds, without chemical bonding or other chemical change, so that each ingredient substance retains its own chemical properties and makeup.
A chemical compound is a substance composed of two or more different elements chemically bonded together in a fixed proportion by mass. However, not all molecules are compounds. Compounds are pure substances that contain two or more elements combined in a definite fixed proportion.
Any substance that contains only one kind of an atom is known as an element. Because atoms cannot be created or destroyed in a chemical reaction, elements such as phosphorus (P4) or sulfur (S8) cannot be broken down into simpler substances by these reactions.
When a molecule is formed from elements of a different species it is a heteroatomic molecule. As an atom is the smallest particle of an element that retains the properties of that element, a molecule is the smallest particle of a compound.
A chemical compound is a substance composed of two or more different elements chemically bonded together in a fixed proportion by mass. However, not all molecules are compounds. Compounds are pure substances that contain two or more elements combined in a definite fixed proportion.
Any substance that contains only one kind of an atom is known as an element. Because atoms cannot be created or destroyed in a chemical reaction, elements such as phosphorus (P4) or sulfur (S8) cannot be broken down into simpler substances by these reactions.
When a molecule is formed from elements of a different species it is a heteroatomic molecule. As an atom is the smallest particle of an element that retains the properties of that element, a molecule is the smallest particle of a compound.
Burning of a candle is an example of:- a)Physical change
- b)Physical and chemical change
- c)Chemical change
- d)Reversible change
Correct answer is option 'B'. Can you explain this answer?
Burning of a candle is an example of:
a)
Physical change
b)
Physical and chemical change
c)
Chemical change
d)
Reversible change
|
|
Ravi Verma answered |
Burning of candle is both physical and chemical change. Burning of candle melts the wax and hence physical state of wax has changed from solid to liquid. Again the wax combines with the atmosphere oxygen and changes to carbon dioxide, heat and light.
Thus both the changes are accompanied in the burning of candle.
A compound is a _______ substance made up of _______ .- a)impure; two or more simpler substances
- b)soft; only one kind of atoms
- c)hard; only one element
- d)pure; two or more elements
Correct answer is option 'D'. Can you explain this answer?
A compound is a _______ substance made up of _______ .
a)
impure; two or more simpler substances
b)
soft; only one kind of atoms
c)
hard; only one element
d)
pure; two or more elements
|
|
Ananya Sharma answered |
A molecule is the smallest particle of a substance that exists independently. Molecules of most elements are made up of only one of atom of that element. Oxygen, along with nitrogen, hydrogen, and chlorine are made up of two atoms.
Which of the following is an example of a pure substance?- a)Air
- b)Brass
- c)Water
- d)Milk
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of a pure substance?
a)
Air
b)
Brass
c)
Water
d)
Milk
|
|
C K Academy answered |
Answer: C. Water
Explanation: Water (H2O) is a pure substance made up entirely of water molecules. Here are some key points:
- Definition: A pure substance contains only one type of particle.
- Composition: Water consists solely of H2O molecules.
- Characteristics: It has consistent properties throughout, such as boiling and freezing points.
Freezing, condensation and evaporation processes are examples of:- a)Chemical change
- b)Physical change
- c)Both physical and chemical change
- d)Neither physical change nor chemical change
Correct answer is option 'B'. Can you explain this answer?
Freezing, condensation and evaporation processes are examples of:
a)
Chemical change
b)
Physical change
c)
Both physical and chemical change
d)
Neither physical change nor chemical change

|
Yajush Srivastava answered |
The correct answer is option B. This is because the process of freezing , condensation and evaporation are the processes by which the state of any substance is changed. So as we all know that the process of state change is always a physical process , so freezing , evaporation and condensation are the examples of the Physical Changes.
.
.
.
If u thought my answer was useful then please upvote my answer
.
.
I hope it was useful
.
.
.
If u thought my answer was useful then please upvote my answer
.
.
I hope it was useful
Which of the following parameters of a substance does not alter during a physical change?- a)State
- b)Mass
- c)Volume
- d)Size
Correct answer is option 'B'. Can you explain this answer?
Which of the following parameters of a substance does not alter during a physical change?
a)
State
b)
Mass
c)
Volume
d)
Size
|
|
Hansa Sharma answered |
A physical change can affect the size, shape or color of a substance but does not affect its composition or mass. The substances may be changed to another phase (i.e. gas, liquid, solid) or separated or combined.
Examples:
when ice melts
when sulfur is mixed with iron filings.
breaking a glass
dissolving sugar in water
when sulfur is mixed with iron filings.
breaking a glass
dissolving sugar in water
Which of the following is an example of a solid in a liquid colloid?- a)Smoke
- b)Shaving cream
- c)Milk of magnesia
- d)Fog
Correct answer is option 'C'. Can you explain this answer?
a)
Smoke
b)
Shaving cream
c)
Milk of magnesia
d)
Fog

|
Divey Sethi answered |
Milk of magnesia is an example of a solid in liquid colloid, where solid particles are dispersed in a liquid medium.
Which of the following is a physical change?
- a)Burning of a Natural Gas
- b)Rusting of iron
- c)Freezing of water
- d)Digestion of food
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a physical change?
a)
Burning of a Natural Gas
b)
Rusting of iron
c)
Freezing of water
d)
Digestion of food
|
|
Mohit Khanna answered |
Physical Change: Freezing of Water
Physical changes are those changes in which the substance undergoes changes in its physical properties like size, shape, state, or color, but its chemical composition remains the same. The freezing of water is a physical change because it only involves a change in the state of matter of water from liquid to solid without any change in its chemical composition.
Explanation:
When water is cooled below zero degree Celsius, it starts losing its heat energy, and the molecules of water start slowing down. As the temperature decreases, the kinetic energy of water molecules decreases and the intermolecular forces take over causing the water molecules to arrange themselves into a specific pattern, forming a crystalline structure, which results in the solidification of water.
The solid ice formed is still water, but the only difference is that its molecules are arranged in such a manner that they form a solid instead of a liquid. This is a reversible process, and the ice can be melted again by heating it above 0 degree Celsius, and it will turn into liquid water.
Conclusion:
Hence, the freezing of water is a physical change as there is no change in the chemical composition of water, and the only change that occurs is a change in the state of matter of water from liquid to solid.
Physical changes are those changes in which the substance undergoes changes in its physical properties like size, shape, state, or color, but its chemical composition remains the same. The freezing of water is a physical change because it only involves a change in the state of matter of water from liquid to solid without any change in its chemical composition.
Explanation:
When water is cooled below zero degree Celsius, it starts losing its heat energy, and the molecules of water start slowing down. As the temperature decreases, the kinetic energy of water molecules decreases and the intermolecular forces take over causing the water molecules to arrange themselves into a specific pattern, forming a crystalline structure, which results in the solidification of water.
The solid ice formed is still water, but the only difference is that its molecules are arranged in such a manner that they form a solid instead of a liquid. This is a reversible process, and the ice can be melted again by heating it above 0 degree Celsius, and it will turn into liquid water.
Conclusion:
Hence, the freezing of water is a physical change as there is no change in the chemical composition of water, and the only change that occurs is a change in the state of matter of water from liquid to solid.
Which of the following is a colloidal solution?
- a)Starch solution
- b)Copper sulphate solution
- c)Chalk powder in water
- d)Kerosene oil and water
Correct answer is option 'A'. Can you explain this answer?
Which of the following is a colloidal solution?
a)
Starch solution
b)
Copper sulphate solution
c)
Chalk powder in water
d)
Kerosene oil and water
|
|
Shubham Sharma answered |
A mixture of water and starch is colloidal because it forms a shell of firmly bound molecules of water that stops the starch particle from aggregating with the molecules of water when they collide.
What type of change takes place when a sodium hydroxide pellet is added to water?- a)Reversible change
- b)Reversible chemical change
- c)Physical change
- d)Chemical change
Correct answer is option 'D'. Can you explain this answer?
What type of change takes place when a sodium hydroxide pellet is added to water?
a)
Reversible change
b)
Reversible chemical change
c)
Physical change
d)
Chemical change
|
|
Vikas Kapoor answered |
Because sodium hydroxide is a type of chemical and when we add it to water than this will be a chemical activity.
If a solution contains 60g of common salt in 340g of water, the mass by mass percentage will be:- a)25 %
- b)15 %
- c)20 %
- d)17.6 %
Correct answer is option 'B'. Can you explain this answer?
If a solution contains 60g of common salt in 340g of water, the mass by mass percentage will be:
a)
25 %
b)
15 %
c)
20 %
d)
17.6 %
|
|
Amit Sharma answered |
Concentration of solution is mass of solute upon mass of solution by 100 so mass of solute is 60g and mass of solvent is 340 so mass of solution is 340+60 is equal to 400 we put these values in formula so 60/400×100=15%.
Which of the following processes describes the direct conversion of a solid into a gas without passing through the liquid state?- a)Fusion
- b)Condensation
- c)Solidification
- d)Sublimation
Correct answer is option 'D'. Can you explain this answer?
Which of the following processes describes the direct conversion of a solid into a gas without passing through the liquid state?
a)
Fusion
b)
Condensation
c)
Solidification
d)
Sublimation

|
Gitanjali Kaur answered |
Understanding Sublimation
Sublimation is a fascinating process that involves the direct transition of a solid into a gas, bypassing the liquid phase entirely. Here’s a detailed breakdown of this process:
Definition of Sublimation
- Sublimation occurs when certain substances, such as dry ice (solid carbon dioxide) or iodine, transition from a solid state directly to a gaseous state when heated.
Characteristics of Sublimation
- No Liquid Phase: The key feature of sublimation is that there is no intermediate liquid form; the solid transforms directly into gas.
- Endothermic Process: Sublimation requires energy, often in the form of heat, which allows the molecules in the solid to gain enough energy to overcome intermolecular forces and escape into the air as gas.
Examples of Sublimation
- Dry Ice: Dry ice sublimes at room temperature, turning directly into carbon dioxide gas, which is commonly used in fog machines and refrigeration.
- Iodine Crystals: When heated, iodine crystals sublimate, producing a purple vapor without forming any liquid.
Importance of Sublimation
- Applications in Industry: Sublimation is used in freeze-drying food, which preserves its structure and nutrients while removing moisture.
- Scientific Observations: Sublimation is also observed in various natural processes and is essential in understanding phase changes in materials.
In summary, sublimation is the process defined in the question, making option 'D' the correct answer. It highlights the unique ability of certain solids to transition directly into gas, a fascinating aspect of physical chemistry.
Sublimation is a fascinating process that involves the direct transition of a solid into a gas, bypassing the liquid phase entirely. Here’s a detailed breakdown of this process:
Definition of Sublimation
- Sublimation occurs when certain substances, such as dry ice (solid carbon dioxide) or iodine, transition from a solid state directly to a gaseous state when heated.
Characteristics of Sublimation
- No Liquid Phase: The key feature of sublimation is that there is no intermediate liquid form; the solid transforms directly into gas.
- Endothermic Process: Sublimation requires energy, often in the form of heat, which allows the molecules in the solid to gain enough energy to overcome intermolecular forces and escape into the air as gas.
Examples of Sublimation
- Dry Ice: Dry ice sublimes at room temperature, turning directly into carbon dioxide gas, which is commonly used in fog machines and refrigeration.
- Iodine Crystals: When heated, iodine crystals sublimate, producing a purple vapor without forming any liquid.
Importance of Sublimation
- Applications in Industry: Sublimation is used in freeze-drying food, which preserves its structure and nutrients while removing moisture.
- Scientific Observations: Sublimation is also observed in various natural processes and is essential in understanding phase changes in materials.
In summary, sublimation is the process defined in the question, making option 'D' the correct answer. It highlights the unique ability of certain solids to transition directly into gas, a fascinating aspect of physical chemistry.
A Substance can be beaten into sheets and beaten into wires. What will you call it?- a)It is both brittle and lustrous
- b)It is both sonorous and ductile
- c)It is both Malleable and ductile
- d)It is both malleable and brittle
Correct answer is option 'C'. Can you explain this answer?
A Substance can be beaten into sheets and beaten into wires. What will you call it?
a)
It is both brittle and lustrous
b)
It is both sonorous and ductile
c)
It is both Malleable and ductile
d)
It is both malleable and brittle
|
|
Raghav Bansal answered |
A substance that can be beaten into thin sheets and drawn into thin wire is both malleable and ductile. Gold is most malleable and ductile element.
Clouds and fogs are example of- a)Gas in liquid solution
- b)Gas in solid solution
- c)Gas in gas solution
- d)Liquid in gas solution
Correct answer is option 'D'. Can you explain this answer?
Clouds and fogs are example of
a)
Gas in liquid solution
b)
Gas in solid solution
c)
Gas in gas solution
d)
Liquid in gas solution

|
Rohit Verma answered |
Clouds and fogs are example of liquid in gas solution. Cloud is formed by combination of water vapour with dust particles. Fogs contain water vapour and gases.
The components of a solution are:- a)Dispersed particles and solvent
- b)Solute and solvent
- c)Dispersed phase and dispersion medium
- d)Solute and dispersed medium
Correct answer is option 'B'. Can you explain this answer?
The components of a solution are:
a)
Dispersed particles and solvent
b)
Solute and solvent
c)
Dispersed phase and dispersion medium
d)
Solute and dispersed medium
|
|
Alok Verma answered |
A solution is a homogeneous mixture of particles (ions, atoms, or molecules). Since it is homogeneous the component parts cannot be easily identified. It is made up of the solute, which is present in lesser amount and the solvent, which is present in greater amount.
Assertion (A): In a saturated solution, no more solute can be dissolved at a given temperature.Reason (R): The solubility of a substance determines the maximum amount of solute that can be dissolved in a solution at a specific temperature.- a)If both Assertion and Reason are true and Reason is the correct explanation of Assertion
- b)If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
- c)If Assertion is true but Reason is false
- d)If both Assertion and Reason are false
Correct answer is option 'B'. Can you explain this answer?
Assertion (A): In a saturated solution, no more solute can be dissolved at a given temperature.
Reason (R): The solubility of a substance determines the maximum amount of solute that can be dissolved in a solution at a specific temperature.
a)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion
b)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
c)
If Assertion is true but Reason is false
d)
If both Assertion and Reason are false
|
|
Zachary Foster answered |
- The Assertion is correct. A saturated solution is one where the maximum amount of solute has been dissolved at a given temperature.
- The Reason is correct. Solubility indeed determines the maximum amount of solute that can be dissolved in a solution at a specific temperature.
- However, the Reason is not the correct explanation of the Assertion. While both statements are individually true, the Reason does not directly explain why a saturated solution cannot dissolve more solute.
- Therefore, the correct option is B: If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
Which of the following statement is always true when a substance undergoes physical change- a)It changes colour
- b)a new substance is formed
- c)It boil
- d)Its composition remains the same
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is always true when a substance undergoes physical change
a)
It changes colour
b)
a new substance is formed
c)
It boil
d)
Its composition remains the same
|
|
Athira Saha answered |
Explanation:
When a substance undergoes a physical change, its composition remains the same. This means that the substance remains chemically unchanged and the same substance can be isolated after the physical change. Physical changes involve a change in the state of matter, size, shape or appearance of the substance without any change in its chemical composition. Examples of physical changes include melting, freezing, boiling, condensation, sublimation, cutting, grinding etc.
Examples:
Conclusion:
Therefore, it can be concluded that the statement "Its composition remains the same" is always true when a substance undergoes physical changes.
When a substance undergoes a physical change, its composition remains the same. This means that the substance remains chemically unchanged and the same substance can be isolated after the physical change. Physical changes involve a change in the state of matter, size, shape or appearance of the substance without any change in its chemical composition. Examples of physical changes include melting, freezing, boiling, condensation, sublimation, cutting, grinding etc.
Examples:
- When ice (solid water) melts, it forms liquid water. The composition of the substance remains the same, only the state of matter has changed.
- When a piece of paper is cut into smaller pieces, its shape and size change but it is still paper with the same chemical composition.
- When water is boiled, it forms steam (water vapour). Again, only the state of matter has changed and the chemical composition remains the same.
Conclusion:
Therefore, it can be concluded that the statement "Its composition remains the same" is always true when a substance undergoes physical changes.
Which of the following statement is true about compounds?- a)Compound is homogeneous and have fixed composition
- b)Compound is heterogeneous and have fixed composition
- c)Compound is heterogeneous
- d)Compound is heterogeneous and has no fixed composition
Correct answer is option 'A'. Can you explain this answer?
Which of the following statement is true about compounds?
a)
Compound is homogeneous and have fixed composition
b)
Compound is heterogeneous and have fixed composition
c)
Compound is heterogeneous
d)
Compound is heterogeneous and has no fixed composition
|
|
Divya Sriram answered |
Compounds are homogeneous as they have uniform composition through out. They also have a fixed composition. Hence, the answer is option A.
A solution is a ______ mixture of two or more substances.- a)Heterogeneous
- b)Homogeneous
- c)Transparent
- d)Opaque
Correct answer is option 'B'. Can you explain this answer?
A solution is a ______ mixture of two or more substances.
a)
Heterogeneous
b)
Homogeneous
c)
Transparent
d)
Opaque
|
|
Zachary Foster answered |
A solution is a homogeneous mixture because all the particles are evenly spread out, making it look the same everywhere in the mixture. Imagine mixing sugar in water until you can't see the sugar anymore. That's a homogeneous solution where everything looks the same.
Which of the following has the smell of rotten egg?- a)Iron Sulphide
- b)Calcium Sulphide
- c)Hydrogen Sulphide
- d)Copper Sulphide
Correct answer is option 'C'. Can you explain this answer?
Which of the following has the smell of rotten egg?
a)
Iron Sulphide
b)
Calcium Sulphide
c)
Hydrogen Sulphide
d)
Copper Sulphide

|
Akshara Iyer answered |
Answer:
Hydrogen Sulphide has the smell of rotten egg.
Hydrogen Sulphide is a colorless gas with a characteristic odor that resembles the smell of rotten eggs or sewage. It is commonly produced by the breakdown of organic matter that contains sulfur, such as decaying plants and animal waste. The presence of hydrogen sulphide gas is often associated with the process of decay and decomposition.
Iron Sulphide, also known as pyrite or fool's gold, does not have the smell of rotten egg. It is a mineral that is commonly found in sedimentary rocks and can sometimes have a metallic odor, but it is not the same as the smell of rotten eggs.
Calcium Sulphide does not have the smell of rotten egg either. It is a chemical compound that is often used in the production of dyes and pigments. It has a characteristic odor, but it is not the same as the smell of rotten eggs.
Copper Sulphide does not have the smell of rotten egg either. It is a compound that is commonly used in the production of various products, including electrical wires and batteries. It does not have a strong odor and is not associated with the smell of rotten eggs.
In conclusion, Hydrogen Sulphide is the compound that has the smell of rotten egg. It is a colorless gas that is often produced during the decay of organic matter and is commonly associated with the smell of rotten eggs or sewage.
Hydrogen Sulphide has the smell of rotten egg.
Hydrogen Sulphide is a colorless gas with a characteristic odor that resembles the smell of rotten eggs or sewage. It is commonly produced by the breakdown of organic matter that contains sulfur, such as decaying plants and animal waste. The presence of hydrogen sulphide gas is often associated with the process of decay and decomposition.
Iron Sulphide, also known as pyrite or fool's gold, does not have the smell of rotten egg. It is a mineral that is commonly found in sedimentary rocks and can sometimes have a metallic odor, but it is not the same as the smell of rotten eggs.
Calcium Sulphide does not have the smell of rotten egg either. It is a chemical compound that is often used in the production of dyes and pigments. It has a characteristic odor, but it is not the same as the smell of rotten eggs.
Copper Sulphide does not have the smell of rotten egg either. It is a compound that is commonly used in the production of various products, including electrical wires and batteries. It does not have a strong odor and is not associated with the smell of rotten eggs.
In conclusion, Hydrogen Sulphide is the compound that has the smell of rotten egg. It is a colorless gas that is often produced during the decay of organic matter and is commonly associated with the smell of rotten eggs or sewage.
The particles of suspension - a)can’t be seen with naked eye
- b)can’t be seen with the help of powerful microscope
- c)can be seen with naked eye
- d)can’t be seen with electron microscope
Correct answer is option 'C'. Can you explain this answer?
The particles of suspension
a)
can’t be seen with naked eye
b)
can’t be seen with the help of powerful microscope
c)
can be seen with naked eye
d)
can’t be seen with electron microscope

|
Anurag Maurya answered |
The particle of suspension is is not shelt down.
it can be seen with tyndall effect
Who was the first scientist to define an element as a basic form of matter that cannot be broken down further by chemical reactions?- a)Robert Boyle
- b)Antoine Lavoisier
- c)John Dalton
- d)Isaac Newton
Correct answer is option 'A'. Can you explain this answer?
Who was the first scientist to define an element as a basic form of matter that cannot be broken down further by chemical reactions?
a)
Robert Boyle
b)
Antoine Lavoisier
c)
John Dalton
d)
Isaac Newton

|
Let's Tute answered |
Robert Boyle was the first scientist to use the term element in 1661. However, it was Antoine Lavoisier, a French chemist, who provided a clear definition of an element. He described an element as a basic form of matter that cannot be broken down into simpler substances through chemical reactions.
Elements can be classified into three main categories:
- Metals
- Non-metals
- Metalloids
Which of the following is true only for elements but not for compounds?- a)They are homogeneous.
- b)They are the simplest substances and cannot be broken down further.
- c)They are formed by a chemical combination of two or more substances.
- d)They can exist in different physical states (solid, liquid, gas).
Correct answer is option 'B'. Can you explain this answer?
Which of the following is true only for elements but not for compounds?
a)
They are homogeneous.
b)
They are the simplest substances and cannot be broken down further.
c)
They are formed by a chemical combination of two or more substances.
d)
They can exist in different physical states (solid, liquid, gas).
|
|
C K Academy answered |
Elements:
- Elements are the simplest form of matter and cannot be broken down into simpler substances by any chemical means.
- They are homogeneous and can exist in different physical states.
Compounds:
- Compounds are formed by a chemical combination of two or more elements and can be broken down into their constituent elements.
- Compounds are also homogeneous but are not the simplest substances.
Thus, the statement "They are the simplest substances and cannot be broken down further" applies only to elements.
What is the name given to the liquid which contains in it some suspended particles?- a)Collided solution
- b)Suspension
- c)True solution
- d)All of these
Correct answer is option 'B'. Can you explain this answer?
What is the name given to the liquid which contains in it some suspended particles?
a)
Collided solution
b)
Suspension
c)
True solution
d)
All of these

|
Abhishek Datta answered |
The liquid which contains some suspended particles are called suspension. The size of particle is more than 100nm.
How many naturally occurring elements are there?- a)82
- b)92
- c)102
- d)112
Correct answer is option 'B'. Can you explain this answer?
How many naturally occurring elements are there?
a)
82
b)
92
c)
102
d)
112

|
Imk Pathshala answered |
There are 92 naturally occurring elements. These elements exist in nature and are not artificially created. Here are some key points about these elements:
- The majority of elements are solid.
- Eleven elements are gases at room temperature.
- Two elements, mercury and bromine, are liquid at room temperature.
- Elements such as gallium and cesium become liquid just above room temperature.
In addition to the naturally occurring elements, there are also man-made elements, which are typically created in laboratories or nuclear reactors.
State whether the following statement is True or FalseA suspension is a mixture where solute particles dissolve completely in the liquid.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
State whether the following statement is True or False
A suspension is a mixture where solute particles dissolve completely in the liquid.
a)
True
b)
False
|
|
Abhijeet Kumar answered |
Explanation:
Definition of Suspension:
- A suspension is a heterogeneous mixture in which solute particles do not dissolve completely in the liquid.
- The particles in a suspension are large enough to settle out over time.
Dissolution in Suspensions:
- In a suspension, the solute particles are typically insoluble or only partially soluble in the liquid.
- These particles remain suspended in the liquid without dissolving completely.
Characteristics of Suspensions:
- Suspensions are cloudy or opaque due to the presence of undissolved particles.
- They may require shaking or stirring to temporarily mix the components, as the particles will eventually settle out.
Examples of Suspensions:
- Common examples of suspensions include muddy water, paint, and certain medicines like antacid liquids.
Conclusion:
In conclusion, the statement that a suspension is a mixture where solute particles dissolve completely in the liquid is false. Suspensions are actually mixtures where solute particles do not dissolve completely in the liquid, and instead remain suspended in the liquid as undissolved particles.
Definition of Suspension:
- A suspension is a heterogeneous mixture in which solute particles do not dissolve completely in the liquid.
- The particles in a suspension are large enough to settle out over time.
Dissolution in Suspensions:
- In a suspension, the solute particles are typically insoluble or only partially soluble in the liquid.
- These particles remain suspended in the liquid without dissolving completely.
Characteristics of Suspensions:
- Suspensions are cloudy or opaque due to the presence of undissolved particles.
- They may require shaking or stirring to temporarily mix the components, as the particles will eventually settle out.
Examples of Suspensions:
- Common examples of suspensions include muddy water, paint, and certain medicines like antacid liquids.
Conclusion:
In conclusion, the statement that a suspension is a mixture where solute particles dissolve completely in the liquid is false. Suspensions are actually mixtures where solute particles do not dissolve completely in the liquid, and instead remain suspended in the liquid as undissolved particles.
Which property is NOT a characteristic of a solution?- a)Homogeneous mixture
- b)Particles scatter light
- c)Particles do not settle down
- d)Particles cannot be separated by filtration
Correct answer is option 'B'. Can you explain this answer?
a)
Homogeneous mixture
b)
Particles scatter light
c)
Particles do not settle down
d)
Particles cannot be separated by filtration
|
|
Maitri Choudhury answered |
Understanding Solutions
A solution is a homogeneous mixture composed of two or more substances. To clarify why option 'B' is not a characteristic of a solution, let’s examine the properties of solutions in detail.
Characteristics of a Solution:
- **Homogeneous Mixture:**
Solutions are uniform in composition. The solute is completely dissolved in the solvent, making it indistinguishable.
- **Particles Do Not Settle Down:**
In a true solution, the particles remain evenly dispersed and do not settle over time. This indicates stability and uniformity.
- **Particles Cannot Be Separated by Filtration:**
The solute particles are at the molecular or ionic level, making them too small to be separated from the solvent through filtration.
Why Option 'B' is Incorrect:
- **Particles Scatter Light:**
This property is **not** characteristic of solutions. In fact, solutions do not exhibit the Tyndall effect, which is the scattering of light caused by larger particles.
- **Colloids vs. Solutions:**
The scattering of light is a property of colloidal mixtures, where larger particles are present. Solutions, having particles that are molecular in size, do not scatter light and thus appear clear.
In summary, the correct answer is option 'B' because solutions do not scatter light, distinguishing them from other types of mixtures like colloids.
A solution is a homogeneous mixture composed of two or more substances. To clarify why option 'B' is not a characteristic of a solution, let’s examine the properties of solutions in detail.
Characteristics of a Solution:
- **Homogeneous Mixture:**
Solutions are uniform in composition. The solute is completely dissolved in the solvent, making it indistinguishable.
- **Particles Do Not Settle Down:**
In a true solution, the particles remain evenly dispersed and do not settle over time. This indicates stability and uniformity.
- **Particles Cannot Be Separated by Filtration:**
The solute particles are at the molecular or ionic level, making them too small to be separated from the solvent through filtration.
Why Option 'B' is Incorrect:
- **Particles Scatter Light:**
This property is **not** characteristic of solutions. In fact, solutions do not exhibit the Tyndall effect, which is the scattering of light caused by larger particles.
- **Colloids vs. Solutions:**
The scattering of light is a property of colloidal mixtures, where larger particles are present. Solutions, having particles that are molecular in size, do not scatter light and thus appear clear.
In summary, the correct answer is option 'B' because solutions do not scatter light, distinguishing them from other types of mixtures like colloids.
Chapter doubts & questions for Is Matter Around Us Pure? - Chemistry for Grade 9 2025 is part of Grade 9 exam preparation. The chapters have been prepared according to the Grade 9 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 9 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Is Matter Around Us Pure? - Chemistry for Grade 9 in English & Hindi are available as part of Grade 9 exam.
Download more important topics, notes, lectures and mock test series for Grade 9 Exam by signing up for free.
Chemistry for Grade 9
39 videos|52 docs|29 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









