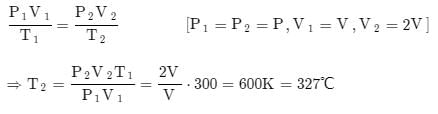All Exams >
Mechanical Engineering >
Mechanical Engineering SSC JE (Technical) >
All Questions
All questions of Thermodynamics for Mechanical Engineering Exam
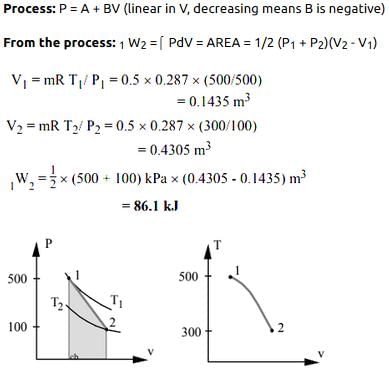
A piston cylinder contains 0.5 kg of air at 500 kPa and 500 K. The air expands in a process so pressure is linearly decreasing with volume to a final state of 100 kPa and 300 K. Find the work in the process.- a)56.1 kJ
- b)66.1 kJ
- c)76.1 kJ
- d)86.1 kJ
Correct answer is option 'D'. Can you explain this answer?
A piston cylinder contains 0.5 kg of air at 500 kPa and 500 K. The air expands in a process so pressure is linearly decreasing with volume to a final state of 100 kPa and 300 K. Find the work in the process.
a)
56.1 kJ
b)
66.1 kJ
c)
76.1 kJ
d)
86.1 kJ
|
|
Sarita Yadav answered |
Specific heat of air at constant pressure is equal to
- a)0.17 Btu/lb °F
- b)0.21 Btu/lb °F
- c)0.24 Btu/lb °F
- d)1.0 Btu/lb °F
- e)1.41 Btu/lb °F
Correct answer is 'C'. Can you explain this answer?
Specific heat of air at constant pressure is equal to
a)
0.17 Btu/lb °F
b)
0.21 Btu/lb °F
c)
0.24 Btu/lb °F
d)
1.0 Btu/lb °F
e)
1.41 Btu/lb °F

|
Jalaluddin Mohd Akbar answered |
For ordinary calculations - a value of specific heat cp = 1.0 kJ/kg K (equal to kJ/kg oC) or 0.24 Btu(IT)/lb °F - is normally accurate enough
The value of n = 1 in the polytropic process indicates it to be- a)Reversible process
- b)Adiabatic process
- c)Isothermal process
- d)Irreversible process
- e)at a temperature of – 273ºK
Correct answer is option 'C'. Can you explain this answer?
The value of n = 1 in the polytropic process indicates it to be
a)
Reversible process
b)
Adiabatic process
c)
Isothermal process
d)
Irreversible process
e)
at a temperature of – 273ºK
|
|
Neha Joshi answered |
PVγ = constant
is the expression for Polytropic Process where γ is a constant
If γ = 1, PV = constant which is only true for an isothermal process.
Hence option C is correct.
According to Gay-Lussac law for a perfect gas, the absolute pressure of given mass varies directly as- a)temperature
- b)absolute
- c)absolute temperature, if volume is kept constant
- d)volume, if temperature is kept constant
- e)remains constant, if volume and temperature are kept constant
Correct answer is option 'C'. Can you explain this answer?
According to Gay-Lussac law for a perfect gas, the absolute pressure of given mass varies directly as
a)
temperature
b)
absolute
c)
absolute temperature, if volume is kept constant
d)
volume, if temperature is kept constant
e)
remains constant, if volume and temperature are kept constant

|
Bijoy Kapoor answered |
Gay–Lussac’s Law shows the relationship between the absolute temperature and pressure of a gas. At a fixed volume, the absolute temperature and pressure of a gas are directly proportional to each other.
Superheated vapour behaves- a)exactly as gas
- b)as steam
- c)as ordinary vapour
- d)approximately as a gas
- e)as average of gas and vapour.
Correct answer is option 'D'. Can you explain this answer?
Superheated vapour behaves
a)
exactly as gas
b)
as steam
c)
as ordinary vapour
d)
approximately as a gas
e)
as average of gas and vapour.

|
Shivam Sharma answered |
Superheated steam. Superheated steam is a steam at a temperature higher than its vaporization (boiling) point at the absolute pressure where the temperature is measured. ... This is because the superheated steam is dry.
According to Dalton's law the total pressure of the mixture of gases is equal to- a)greater of the partial pressures of all
- b)average of the partial pressure of all
- c)sum of the partial pressures of all
- d)sum of the partial pressure of all divided by average molecular weight
- e)atmospheric pressure.
Correct answer is option 'C'. Can you explain this answer?
According to Dalton's law the total pressure of the mixture of gases is equal to
a)
greater of the partial pressures of all
b)
average of the partial pressure of all
c)
sum of the partial pressures of all
d)
sum of the partial pressure of all divided by average molecular weight
e)
atmospheric pressure.
|
|
Om Desai answered |
Dalton's law (also called Dalton's law of partial pressures) states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases.
Characteristic gas constant of a gas is equal to- a)Cp/Cv
- b)Cv/Cp
- c)Cp – Cv
- d)Cp + Cv
- e)Cp × Cv
Correct answer is option 'C'. Can you explain this answer?
Characteristic gas constant of a gas is equal to
a)
Cp/Cv
b)
Cv/Cp
c)
Cp – Cv
d)
Cp + Cv
e)
Cp × Cv
|
|
Rhea Reddy answered |
We know by the formula Cp - Cv = R
If a gas is heated against a pressure, keeping the volume constant, then work done will be equal to- a)+ v
- b)– ve
- c)zero
- d)pressure × volume
- e)any were between zero and infinity.
Correct answer is option 'C'. Can you explain this answer?
If a gas is heated against a pressure, keeping the volume constant, then work done will be equal to
a)
+ v
b)
– ve
c)
zero
d)
pressure × volume
e)
any were between zero and infinity.
|
|
Lavanya Menon answered |
-If a gas is heated against a pressure, keeping the volume constant, then work done will be equal to zero.
-If the gas is heated at a constant pressure then, The volume of the gas will increase. Now, If volume remains constant during the process of heating, The pressure will increase.
-For a gas, work is the product of the pressure p and the volume V during a change of volume. On a graph of pressure versus volume, the work is the area under the curve that describes how the state is changed from State 1 to State 2.
One kg of carbon monoxide requires __________ kg of oxygen to produce 11/7 kg of carbon dioxide gas.- a)11/4
- b)9/7
- c)7/9
- d)4/7
- e)None of the above
Correct answer is option 'D'. Can you explain this answer?
One kg of carbon monoxide requires __________ kg of oxygen to produce 11/7 kg of carbon dioxide gas.
a)
11/4
b)
9/7
c)
7/9
d)
4/7
e)
None of the above

|
Sreemoyee Deshpande answered |
2CO + O2 = 2 CO2
(2*28) (16*2) (2*44)
(2*28) (16*2) (2*44)
=56 kg =32 kg =88 kg
=1 kg =32/56 kg =88/56 kg
=1 kg =4/7 kg =11/7 kg
That is 4/7 kg of O2 is required for preaparing 11/7 kg of CO2 from 1 kg CO.
An open system is one in which- a)mass does not cross boundaries of the system, though energy may do so
- b)neither mass nor energy crosses the boundaries of the system
- c)both energy and mass cross the boundaries of the system
- d)mass crosses the boundary but not the energy
- e)thermodynamic reactions do not occur.
Correct answer is option 'C'. Can you explain this answer?
An open system is one in which
a)
mass does not cross boundaries of the system, though energy may do so
b)
neither mass nor energy crosses the boundaries of the system
c)
both energy and mass cross the boundaries of the system
d)
mass crosses the boundary but not the energy
e)
thermodynamic reactions do not occur.

|
Omkar Lambe answered |
Steam turbine is a perfect example for an open loop system.
It consist of conversion of steam into energy across the boiler.
Also mass is transfered.
It consist of conversion of steam into energy across the boiler.
Also mass is transfered.
Kinetic energy of the molecules in terms of absolute temperature (T) is proportional to- a)T
- b)1/T
- c)T2
- d)
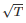
- e)
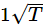
Correct answer is option 'A'. Can you explain this answer?
Kinetic energy of the molecules in terms of absolute temperature (T) is proportional to
a)
T
b)
1/T
c)
T2
d)
e)
|
|
Om Desai answered |
Kinetic energy of molecules is the form of internal energy in a closed vessel, and internal energy is the function of temperature so kinetic energy of molecules is proportional to absolute temprature
Which of the following variables controls the physical properties of a perfect gas- a)pressure
- b)temperature
- c)volume
- d)all of the above
- e)atomic mass
Correct answer is option 'D'. Can you explain this answer?
Which of the following variables controls the physical properties of a perfect gas
a)
pressure
b)
temperature
c)
volume
d)
all of the above
e)
atomic mass

|
Bijoy Kapoor answered |
Perfect gas, also called ideal gas, a gas that conforms, in physical behaviour, to a particular, idealized relation between pressure, volume, and temperature called the general gas law. This law is a generalization containing both Boyle’s law and Charles’s law as special cases and states that for a specified quantity of gas, the product of the volume v and pressure p is proportional to the absolute temperature t; i.e., in equation form, pv = kt, in which k is a constant. Such a relation for a substance is called its equation of state and is sufficient to describe its gross behaviour.
Gases have- a)only one value of specific heat
- b)two values of specific heat
- c)three values of specific heat
- d)no value of specific heat
- e)under some conditions one value and sometimes two values of specific heat
Correct answer is option 'B'. Can you explain this answer?
Gases have
a)
only one value of specific heat
b)
two values of specific heat
c)
three values of specific heat
d)
no value of specific heat
e)
under some conditions one value and sometimes two values of specific heat
|
|
Rajeev Sharma answered |
A solid or a liquid when heated, does not undergo any change in the volume or pressure. But in case of a gas, both the pressure and volume change on heating. Therefore, specific heat of a gas is defined either at constant volume or at constant pressure and hence a gas has two specific heats.
According to avogadro's law, for a given pressure and temperature, each molecule of a gas- a)occupies volume proportional to its molecular weight
- b)occupies volume proportional to its specific weight
- c)occupies volume inversely proportional to its molecular weight
- d)occupies volume inversely proportional to its specific weight
- e)occupies same volume
Correct answer is option 'A'. Can you explain this answer?
According to avogadro's law, for a given pressure and temperature, each molecule of a gas
a)
occupies volume proportional to its molecular weight
b)
occupies volume proportional to its specific weight
c)
occupies volume inversely proportional to its molecular weight
d)
occupies volume inversely proportional to its specific weight
e)
occupies same volume
|
|
Shruti Bose answered |
Avogadro's law is a fundamental gas law that states that, for a given pressure and temperature, equal volumes of gases contain the same number of molecules. This law helps to understand the behavior of gases and is widely used in various fields of science and engineering. The law can be mathematically expressed as follows:
V ∝ n
where V is the volume, n is the number of moles of gas, and ∝ means "proportional to." In other words, the volume of a gas is directly proportional to the number of molecules in it, assuming constant temperature and pressure.
Explanation:
Avogadro's law implies that two gases with the same number of molecules will occupy the same volume at the same temperature and pressure. However, different gases have different molecular weights, which means that the volume occupied by a gas is proportional to its molecular weight.
In other words, heavier gases will occupy more volume than lighter gases, assuming the same number of molecules, temperature, and pressure. This is because the molecular weight of a gas is directly related to the size and mass of its molecules, which affects the space they occupy.
Therefore, option A is the correct answer. Each molecule of a gas occupies a volume proportional to its molecular weight, according to Avogadro's law. This means that heavier gases will occupy more volume than lighter gases, assuming the same number of molecules, temperature, and pressure.
Conclusion:
Avogadro's law is essential to understand the behavior of gases and their properties. The law states that, for a given pressure and temperature, equal volumes of gases contain the same number of molecules. Furthermore, the volume occupied by a gas is proportional to its molecular weight, which means that heavier gases will occupy more volume than lighter gases.
V ∝ n
where V is the volume, n is the number of moles of gas, and ∝ means "proportional to." In other words, the volume of a gas is directly proportional to the number of molecules in it, assuming constant temperature and pressure.
Explanation:
Avogadro's law implies that two gases with the same number of molecules will occupy the same volume at the same temperature and pressure. However, different gases have different molecular weights, which means that the volume occupied by a gas is proportional to its molecular weight.
In other words, heavier gases will occupy more volume than lighter gases, assuming the same number of molecules, temperature, and pressure. This is because the molecular weight of a gas is directly related to the size and mass of its molecules, which affects the space they occupy.
Therefore, option A is the correct answer. Each molecule of a gas occupies a volume proportional to its molecular weight, according to Avogadro's law. This means that heavier gases will occupy more volume than lighter gases, assuming the same number of molecules, temperature, and pressure.
Conclusion:
Avogadro's law is essential to understand the behavior of gases and their properties. The law states that, for a given pressure and temperature, equal volumes of gases contain the same number of molecules. Furthermore, the volume occupied by a gas is proportional to its molecular weight, which means that heavier gases will occupy more volume than lighter gases.
One volume basis, air contains following parts of oxygen- a)21
- b)23
- c)25
- d)77
- e)79
Correct answer is option 'A'. Can you explain this answer?
One volume basis, air contains following parts of oxygen
a)
21
b)
23
c)
25
d)
77
e)
79
|
|
Ananya Kumari answered |
One volume basis, air contain following parts of oxygen,
21 volume of oxygen ans
21 volume of oxygen ans
Which of the following quantities do not represent the property of the system.- a)òpdv
- b)òvdp
- c)cyclic òpdv
- d)cyclic òvdp
- e)none of the above
Correct answer is option 'C'. Can you explain this answer?
Which of the following quantities do not represent the property of the system.
a)
òpdv
b)
òvdp
c)
cyclic òpdv
d)
cyclic òvdp
e)
none of the above
|
|
Sharmila Chauhan answered |
Explanation:
In thermodynamics, there are properties and processes. Properties are the state variables that depend only on the current state of the system and not on the process used to achieve that state. Whereas, processes are the ways in which a system can change from one state to another state.
a)pdv and b)vdp are the expressions of the first law of thermodynamics, which relates the changes in internal energy of a system to the work done on the system and the heat added to the system.
c)cyclic pdv and d)cyclic vdp are the expressions of cyclic processes, which are processes that start and end at the same state, and have a net change of zero in all the state variables. Cyclic processes are important because they allow the system to perform work without a net change in any state variable.
Therefore, the answer is option 'C', which represents a cyclic process and not a property of the system. The cyclic process involves a closed loop, which starts and ends at the same state, and therefore, the net change in volume is zero. As a result, cyclic pdv is also zero and does not represent a property of the system.
Conclusion:
In thermodynamics, it is important to distinguish between properties and processes. Properties are the state variables that depend only on the current state of the system and not on the process used to achieve that state. Processes are the ways in which a system can change from one state to another state. Cyclic processes are important because they allow the system to perform work without a net change in any state variable.
In thermodynamics, there are properties and processes. Properties are the state variables that depend only on the current state of the system and not on the process used to achieve that state. Whereas, processes are the ways in which a system can change from one state to another state.
a)pdv and b)vdp are the expressions of the first law of thermodynamics, which relates the changes in internal energy of a system to the work done on the system and the heat added to the system.
c)cyclic pdv and d)cyclic vdp are the expressions of cyclic processes, which are processes that start and end at the same state, and have a net change of zero in all the state variables. Cyclic processes are important because they allow the system to perform work without a net change in any state variable.
Therefore, the answer is option 'C', which represents a cyclic process and not a property of the system. The cyclic process involves a closed loop, which starts and ends at the same state, and therefore, the net change in volume is zero. As a result, cyclic pdv is also zero and does not represent a property of the system.
Conclusion:
In thermodynamics, it is important to distinguish between properties and processes. Properties are the state variables that depend only on the current state of the system and not on the process used to achieve that state. Processes are the ways in which a system can change from one state to another state. Cyclic processes are important because they allow the system to perform work without a net change in any state variable.
Which of the following items is not a path function- a)heat
- b)work
- c)kinetic energy
- d)Power
- e)thermal conductivity.
Correct answer is option 'E'. Can you explain this answer?
Which of the following items is not a path function
a)
heat
b)
work
c)
kinetic energy
d)
Power
e)
thermal conductivity.
|
|
Dishani Desai answered |
Path Functions in Thermodynamics
Path functions are the thermodynamic properties that define the state of a system. They depend on the path followed during a process to reach a given state. Some examples of path functions include:
- Work (W): This is the energy transferred to or from a system due to a force acting on it. It is given by the integral of the force with respect to the displacement, and depends on the path followed during the process. Work is a path function because the amount of work done depends on the path taken to go from the initial to the final state.
- Heat (Q): This is the energy transferred to or from a system due to a temperature difference between the system and its surroundings. Heat is also a path function because the amount of heat transferred depends on the path taken during the process.
- Internal Energy (U): This is the sum of the kinetic and potential energies of the molecules in a system. It is a state function because it only depends on the initial and final states of the system, not on the path taken to get there.
- Enthalpy (H): This is the sum of the internal energy and the product of pressure and volume. It is also a state function.
- Entropy (S): This is a measure of the disorder or randomness of a system. It is a state function.
- Gibbs Free Energy (G): This is the energy available to do work in a system at constant temperature and pressure. It is a state function.
Not a Path Function
- Thermal Conductivity: This is a physical property of a material that describes its ability to conduct heat. It is not a path function because it does not depend on the path taken during a process, but rather on the properties of the material itself.
Path functions are the thermodynamic properties that define the state of a system. They depend on the path followed during a process to reach a given state. Some examples of path functions include:
- Work (W): This is the energy transferred to or from a system due to a force acting on it. It is given by the integral of the force with respect to the displacement, and depends on the path followed during the process. Work is a path function because the amount of work done depends on the path taken to go from the initial to the final state.
- Heat (Q): This is the energy transferred to or from a system due to a temperature difference between the system and its surroundings. Heat is also a path function because the amount of heat transferred depends on the path taken during the process.
- Internal Energy (U): This is the sum of the kinetic and potential energies of the molecules in a system. It is a state function because it only depends on the initial and final states of the system, not on the path taken to get there.
- Enthalpy (H): This is the sum of the internal energy and the product of pressure and volume. It is also a state function.
- Entropy (S): This is a measure of the disorder or randomness of a system. It is a state function.
- Gibbs Free Energy (G): This is the energy available to do work in a system at constant temperature and pressure. It is a state function.
Not a Path Function
- Thermal Conductivity: This is a physical property of a material that describes its ability to conduct heat. It is not a path function because it does not depend on the path taken during a process, but rather on the properties of the material itself.
The more effective way of increasing efficiency of Carnot engine is to- a)increase higher temperature
- b)decrease higher temperature
- c)increase lower temperature
- d)decrease lower temperature
- e)keep lower temperature constant.
Correct answer is option 'D'. Can you explain this answer?
The more effective way of increasing efficiency of Carnot engine is to
a)
increase higher temperature
b)
decrease higher temperature
c)
increase lower temperature
d)
decrease lower temperature
e)
keep lower temperature constant.
|
|
Tanvi Shah answered |
Correct Answer :- d
Explanation : Efficiency of carnot engine is increased by decreasing the lower temp and increasing higher temperature but more effects on efficiency of cannot cycle by decreasing temperature as compare increase temperature of cannot engine.
The behaviour of gases can be fully determined by- a)1 law
- b)2 laws
- c)3 laws
- d)4 laws
- e)5 laws
Correct answer is option 'D'. Can you explain this answer?
The behaviour of gases can be fully determined by
a)
1 law
b)
2 laws
c)
3 laws
d)
4 laws
e)
5 laws
|
|
Aditya Deshmukh answered |
Explanation:
- Avogadro's Law states that equal volumes of all ideal gases (at the same temperature and pressure) contain the same number of molecules.
- Boyle's Law states that equal pressure is inversely proportional to volume (when temperature is constant).
- Charles's Law states that volume is proportional to temperature (when pressure is constant). Remember that temperature must be measured in Kelvin.
- Gay-Lussac's Law states that pressure is proportional to temperature (when volume is constant).
You can study basics about Thermodynamics through the document:
Work done in a free expansion process is- a)+ ve
- b)– ve
- c)zero
- d)maximum
- e)minimum
Correct answer is option 'C'. Can you explain this answer?
Work done in a free expansion process is
a)
+ ve
b)
– ve
c)
zero
d)
maximum
e)
minimum

|
Anirban Khanna answered |
During free expansion, no work is done by the gas. The gas goes through states of no thermodynamic equilibrium before reaching its final state, which implies that one cannot define thermodynamic parameters as values of the gas as a whole. So work done in a free expansion process is Zero.
The pressure of a gas in terms of its mean kinetic energy per unit volume E is equal to- a)E/3
- b)E/2
- c)3E/4
- d)2E/3
- e)5E/4
Correct answer is option 'D'. Can you explain this answer?
The pressure of a gas in terms of its mean kinetic energy per unit volume E is equal to
a)
E/3
b)
E/2
c)
3E/4
d)
2E/3
e)
5E/4
|
|
Rahul Bansal answered |
Pressure in terms of kinetic energy per unit volume:- The pressure of a gas is equal to twothird of kinetic energy per unit volume of the gas.
P = 2E/3
Gas laws are applicable to- a)gases as well as vapours
- b)gases and vapours under certain conditions
- c)gases alone and not to vapours
- d)gases and steam
- e)steam and vapours
Correct answer is option 'C'. Can you explain this answer?
Gas laws are applicable to
a)
gases as well as vapours
b)
gases and vapours under certain conditions
c)
gases alone and not to vapours
d)
gases and steam
e)
steam and vapours

|
Baishali Chopra answered |
Gas laws are applicable to gases alone and not to vapours.
Gas Laws and their Applicability
Gas laws are a set of fundamental principles that describe the behavior of gases. These laws include Boyle's law, Charles's law, Gay-Lussac's law, and Avogadro's law. These laws are applicable to gases under certain conditions. Let us understand the applicability of gas laws in detail.
Boyle's Law
Boyle's law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. This law is applicable to gases alone and not to vapours. Vapours are the gases in their liquid or solid form and do not behave like ideal gases.
Charles's Law
Charles's law states that at a constant pressure, the volume of a gas is directly proportional to its temperature. This law is applicable to gases alone and not to vapours. Vapours do not behave like ideal gases, and their volume is affected by the intermolecular forces between the molecules.
Gay-Lussac's Law
Gay-Lussac's law states that at a constant volume, the pressure of a gas is directly proportional to its temperature. This law is applicable to gases alone and not to vapours. Vapours do not behave like ideal gases, and their pressure is affected by the intermolecular forces between the molecules.
Avogadro's Law
Avogadro's law states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. This law is applicable to gases alone and not to vapours. Vapours do not behave like ideal gases, and their molecular composition is affected by the intermolecular forces between the molecules.
Conclusion
In conclusion, gas laws are applicable to gases alone and not to vapours. Vapours do not behave like ideal gases, and their behavior is affected by the intermolecular forces between the molecules. Therefore, it is important to distinguish between gases and vapours when applying gas laws.
Gas Laws and their Applicability
Gas laws are a set of fundamental principles that describe the behavior of gases. These laws include Boyle's law, Charles's law, Gay-Lussac's law, and Avogadro's law. These laws are applicable to gases under certain conditions. Let us understand the applicability of gas laws in detail.
Boyle's Law
Boyle's law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. This law is applicable to gases alone and not to vapours. Vapours are the gases in their liquid or solid form and do not behave like ideal gases.
Charles's Law
Charles's law states that at a constant pressure, the volume of a gas is directly proportional to its temperature. This law is applicable to gases alone and not to vapours. Vapours do not behave like ideal gases, and their volume is affected by the intermolecular forces between the molecules.
Gay-Lussac's Law
Gay-Lussac's law states that at a constant volume, the pressure of a gas is directly proportional to its temperature. This law is applicable to gases alone and not to vapours. Vapours do not behave like ideal gases, and their pressure is affected by the intermolecular forces between the molecules.
Avogadro's Law
Avogadro's law states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. This law is applicable to gases alone and not to vapours. Vapours do not behave like ideal gases, and their molecular composition is affected by the intermolecular forces between the molecules.
Conclusion
In conclusion, gas laws are applicable to gases alone and not to vapours. Vapours do not behave like ideal gases, and their behavior is affected by the intermolecular forces between the molecules. Therefore, it is important to distinguish between gases and vapours when applying gas laws.
Which of the following parameters is constant for a mole for most of the gases at a given temperature and pressure- a)enthalpy
- b)volume
- c)mass
- d)entropy
- e)specific volume
Correct answer is option 'E'. Can you explain this answer?
Which of the following parameters is constant for a mole for most of the gases at a given temperature and pressure
a)
enthalpy
b)
volume
c)
mass
d)
entropy
e)
specific volume
|
|
Anmol Saini answered |
Constant Parameter for a Mole of Gases
The behavior of gases is usually studied in terms of a mole, which is a specific number of particles. At a given temperature and pressure, most gases behave similarly, and they can be described by a few parameters.
The parameter that is constant for a mole of most gases at a given temperature and pressure is the specific volume. Specific volume is defined as the volume of a substance per unit mass, and for gases, it is usually expressed in cubic meters per kilogram (m3/kg).
Explanation
The specific volume of a gas depends on its temperature, pressure, and the number of particles present. However, for a given temperature and pressure, the specific volume of most gases is nearly constant. This is because, at a fixed temperature and pressure, the volume occupied by a gas is directly proportional to the number of particles present in it, and this is known as Avogadro's law.
As a result, the specific volume of a mole of gas at a given temperature and pressure is fixed, and it is the same for most gases. For example, at standard temperature and pressure (STP), which is 0°C and 1 atm, the specific volume of a mole of any gas is approximately 24.45 m3/kmol.
The specific volume of a gas is an essential parameter in many engineering applications, including the design of compressors, turbines, and heat exchangers. It is also used to calculate the density of a gas, which is an important property in gas dynamics.
Conclusion
In conclusion, the specific volume is the parameter that is constant for a mole of most gases at a given temperature and pressure. This parameter is essential in describing the behavior of gases, and it is used in many engineering applications.
The behavior of gases is usually studied in terms of a mole, which is a specific number of particles. At a given temperature and pressure, most gases behave similarly, and they can be described by a few parameters.
The parameter that is constant for a mole of most gases at a given temperature and pressure is the specific volume. Specific volume is defined as the volume of a substance per unit mass, and for gases, it is usually expressed in cubic meters per kilogram (m3/kg).
Explanation
The specific volume of a gas depends on its temperature, pressure, and the number of particles present. However, for a given temperature and pressure, the specific volume of most gases is nearly constant. This is because, at a fixed temperature and pressure, the volume occupied by a gas is directly proportional to the number of particles present in it, and this is known as Avogadro's law.
As a result, the specific volume of a mole of gas at a given temperature and pressure is fixed, and it is the same for most gases. For example, at standard temperature and pressure (STP), which is 0°C and 1 atm, the specific volume of a mole of any gas is approximately 24.45 m3/kmol.
The specific volume of a gas is an essential parameter in many engineering applications, including the design of compressors, turbines, and heat exchangers. It is also used to calculate the density of a gas, which is an important property in gas dynamics.
Conclusion
In conclusion, the specific volume is the parameter that is constant for a mole of most gases at a given temperature and pressure. This parameter is essential in describing the behavior of gases, and it is used in many engineering applications.
To convert volumetric analysis to gravimetric analysis, the relative volume of each constituent of the flue gases is- a)divided by its molecular weight
- b)multiplied by its molecular weight
- c)multiplied by its density
- d)multiplied by its specific weight
- e)divided by its specific weight.
Correct answer is option 'B'. Can you explain this answer?
To convert volumetric analysis to gravimetric analysis, the relative volume of each constituent of the flue gases is
a)
divided by its molecular weight
b)
multiplied by its molecular weight
c)
multiplied by its density
d)
multiplied by its specific weight
e)
divided by its specific weight.

|
Arshiya Mehta answered |
The correct answer is OPTION B.
Convert volumetric to gravitational, relative volume of each constituent of flue gases is multiplied by its molecular weight.
Absolute zero pressure will occur- a)at seal level
- b)at the centre of the earth
- c)when molecular momentum of the system becomes zero
- d)under vacuum conditions
- e)at a temperature of – 273°K
Correct answer is option 'C'. Can you explain this answer?
Absolute zero pressure will occur
a)
at seal level
b)
at the centre of the earth
c)
when molecular momentum of the system becomes zero
d)
under vacuum conditions
e)
at a temperature of – 273°K
|
|
Tanvi Shah answered |
Absolute zero. Absolute zero, temperature at which a thermodynamic system has the lowest energy. It corresponds to −273.15 degreeC on the Celsius temperature scale and to −459.67 degreeF on the Fahrenheit temperature scale.
No liquid can exist as liquid at- a)–273°K
- b)vacuum
- c)zero pressure
- d)centre of earth
- e)in space
Correct answer is option 'C'. Can you explain this answer?
No liquid can exist as liquid at
a)
–273°K
b)
vacuum
c)
zero pressure
d)
centre of earth
e)
in space
|
|
Aditya Deshmukh answered |
No. While most liquids have fairly low binding energies (and thus, relatively high vapor pressures) there are liquids that will not evaporate in a vacuum, one example being ionic liquids.
An expansion process as per law pV = constant is known as- a)parabolic expansion
- b)hyperbolic expansion
- c)isentropic expansion
- d)adiabatic expansion
- e)free expansion
Correct answer is option 'B'. Can you explain this answer?
An expansion process as per law pV = constant is known as
a)
parabolic expansion
b)
hyperbolic expansion
c)
isentropic expansion
d)
adiabatic expansion
e)
free expansion

|
Bhavya Rane answered |
The correct answer to the given question is option B: hyperbolic expansion.
Explanation:
Expansion processes in thermodynamics are classified based on the relationship between pressure (P) and volume (V) during the process. The given relationship, pV = constant, represents a hyperbolic expansion process.
Let's understand the different types of expansion processes:
1. Parabolic Expansion: In a parabolic expansion process, the relationship between pressure and volume is given by pV^2 = constant. This type of expansion is not represented by the given equation pV = constant.
2. Hyperbolic Expansion: In a hyperbolic expansion process, the relationship between pressure and volume is given by pV = constant. This is the equation stated in the question, and it represents a hyperbolic expansion process.
3. Isentropic Expansion: An isentropic expansion process is characterized by constant entropy. The relationship between pressure and volume during an isentropic expansion process is not necessarily pV = constant. Therefore, option C is incorrect.
4. Adiabatic Expansion: An adiabatic expansion process is characterized by no heat transfer between the system and its surroundings. The relationship between pressure and volume during an adiabatic expansion process is given by pV^γ = constant, where γ is the ratio of specific heats. Therefore, option D is incorrect.
5. Free Expansion: A free expansion process occurs when a gas expands into a vacuum without any external work being done. In this case, the gas expands freely and the pressure and volume relationship is not defined by any specific equation. Therefore, option E is incorrect.
In conclusion, the correct answer is option B: hyperbolic expansion, as it represents the given equation pV = constant.
Explanation:
Expansion processes in thermodynamics are classified based on the relationship between pressure (P) and volume (V) during the process. The given relationship, pV = constant, represents a hyperbolic expansion process.
Let's understand the different types of expansion processes:
1. Parabolic Expansion: In a parabolic expansion process, the relationship between pressure and volume is given by pV^2 = constant. This type of expansion is not represented by the given equation pV = constant.
2. Hyperbolic Expansion: In a hyperbolic expansion process, the relationship between pressure and volume is given by pV = constant. This is the equation stated in the question, and it represents a hyperbolic expansion process.
3. Isentropic Expansion: An isentropic expansion process is characterized by constant entropy. The relationship between pressure and volume during an isentropic expansion process is not necessarily pV = constant. Therefore, option C is incorrect.
4. Adiabatic Expansion: An adiabatic expansion process is characterized by no heat transfer between the system and its surroundings. The relationship between pressure and volume during an adiabatic expansion process is given by pV^γ = constant, where γ is the ratio of specific heats. Therefore, option D is incorrect.
5. Free Expansion: A free expansion process occurs when a gas expands into a vacuum without any external work being done. In this case, the gas expands freely and the pressure and volume relationship is not defined by any specific equation. Therefore, option E is incorrect.
In conclusion, the correct answer is option B: hyperbolic expansion, as it represents the given equation pV = constant.
Solid and liquids have- a)one value of specific heat
- b)two values of specific heat
- c)three values of specific heat
- d)no value of specific heat
- e)one value under some conditions and two values under other conditions.
Correct answer is option 'A'. Can you explain this answer?
Solid and liquids have
a)
one value of specific heat
b)
two values of specific heat
c)
three values of specific heat
d)
no value of specific heat
e)
one value under some conditions and two values under other conditions.
|
|
Dipika Bose answered |
Specific Heat of Solids and Liquids
Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree Celsius or Kelvin. Solids and liquids have one value of specific heat. Let's understand this in detail.
Explanation:
- Solid and liquids are condensed forms of matter. The particles in these states are closely packed together and have strong intermolecular forces.
- The specific heat of solids and liquids is a measure of the amount of energy required to vibrate these particles and increase their temperature.
- The specific heat of a substance depends on its atomic and molecular structure, as well as its mass and temperature.
- Since the particles in solids and liquids are tightly packed, they require a fixed amount of energy to vibrate and increase their temperature. Hence, solids and liquids have only one value of specific heat.
Conclusion:
In conclusion, solids and liquids have one value of specific heat as they have closely packed particles and strong intermolecular forces. The specific heat of a substance depends on its atomic and molecular structure, mass, and temperature.
Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree Celsius or Kelvin. Solids and liquids have one value of specific heat. Let's understand this in detail.
Explanation:
- Solid and liquids are condensed forms of matter. The particles in these states are closely packed together and have strong intermolecular forces.
- The specific heat of solids and liquids is a measure of the amount of energy required to vibrate these particles and increase their temperature.
- The specific heat of a substance depends on its atomic and molecular structure, as well as its mass and temperature.
- Since the particles in solids and liquids are tightly packed, they require a fixed amount of energy to vibrate and increase their temperature. Hence, solids and liquids have only one value of specific heat.
Conclusion:
In conclusion, solids and liquids have one value of specific heat as they have closely packed particles and strong intermolecular forces. The specific heat of a substance depends on its atomic and molecular structure, mass, and temperature.
When a gas flows through a very long pipe of uniform cross section, the flow is approximately- a)isentropic
- b)isobaric
- c)isothermal
- d)adiabatic
- e)isochoric
Correct answer is option 'C'. Can you explain this answer?
When a gas flows through a very long pipe of uniform cross section, the flow is approximately
a)
isentropic
b)
isobaric
c)
isothermal
d)
adiabatic
e)
isochoric

|
Rahul Chatterjee answered |
Isothermal Flow
In this section a model dealing with gas that flows through a long tube is described. This model has a applicability to situations which occur in a relatively long distance and where heat transfer is relatively rapid so that the temperature can be treated, for engineering purposes, as a constant. For example, this model is applicable when a natural gas flows over several hundreds of meters.
According to kinetic theory of gases, the absolute zero temperature is attained when- a)volume of the gas is zero
- b)pressure of the gas is zero
- c)kinetic energy of the molecules is zero
- d)specific heat of gas is zero
- e)mass is zero.
Correct answer is option 'C'. Can you explain this answer?
According to kinetic theory of gases, the absolute zero temperature is attained when
a)
volume of the gas is zero
b)
pressure of the gas is zero
c)
kinetic energy of the molecules is zero
d)
specific heat of gas is zero
e)
mass is zero.

|
Pallabi Chavan answered |
According to the kinetic theory of gases, the absolute zero temperature is attained when the kinetic energy of the molecules is zero. This concept is based on the understanding that gases consist of a large number of particles (molecules or atoms) that are in constant random motion.
Explanation:
1. Kinetic Theory of Gases:
The kinetic theory of gases describes the behavior of gases based on the motion of their particles. According to this theory, gases are composed of particles (molecules or atoms) that are in constant motion. The theory assumes the following principles:
- Gas particles are in constant random motion, colliding with each other and with the walls of the container.
- The volume of individual gas particles is negligible compared to the volume of the container.
- The collisions between gas particles and the walls of the container are perfectly elastic, meaning there is no loss of energy during the collisions.
2. Relationship between Temperature and Kinetic Energy:
The temperature of a gas is a measure of the average kinetic energy of its particles. As the temperature increases, the average kinetic energy of the gas particles also increases. Conversely, as the temperature decreases, the average kinetic energy decreases.
3. Absolute Zero Temperature:
According to the kinetic theory of gases, absolute zero is the temperature at which the kinetic energy of the gas particles is zero. At this temperature, the gas particles come to a complete stop, and there is no random motion or collisions between the particles.
4. Implications of Absolute Zero Temperature:
- As the kinetic energy of gas particles is directly related to temperature, reaching absolute zero is theoretically impossible. It is the lowest temperature that can be theoretically achieved.
- At temperatures close to absolute zero, gases exhibit unique properties such as superfluidity and superconductivity.
- The concept of absolute zero is essential in understanding the behavior of gases and other systems at extremely low temperatures.
In conclusion, according to the kinetic theory of gases, the absolute zero temperature is attained when the kinetic energy of the gas particles is zero. This temperature represents the lowest possible temperature and has important implications in the study of gases and low-temperature phenomena.
Explanation:
1. Kinetic Theory of Gases:
The kinetic theory of gases describes the behavior of gases based on the motion of their particles. According to this theory, gases are composed of particles (molecules or atoms) that are in constant motion. The theory assumes the following principles:
- Gas particles are in constant random motion, colliding with each other and with the walls of the container.
- The volume of individual gas particles is negligible compared to the volume of the container.
- The collisions between gas particles and the walls of the container are perfectly elastic, meaning there is no loss of energy during the collisions.
2. Relationship between Temperature and Kinetic Energy:
The temperature of a gas is a measure of the average kinetic energy of its particles. As the temperature increases, the average kinetic energy of the gas particles also increases. Conversely, as the temperature decreases, the average kinetic energy decreases.
3. Absolute Zero Temperature:
According to the kinetic theory of gases, absolute zero is the temperature at which the kinetic energy of the gas particles is zero. At this temperature, the gas particles come to a complete stop, and there is no random motion or collisions between the particles.
4. Implications of Absolute Zero Temperature:
- As the kinetic energy of gas particles is directly related to temperature, reaching absolute zero is theoretically impossible. It is the lowest temperature that can be theoretically achieved.
- At temperatures close to absolute zero, gases exhibit unique properties such as superfluidity and superconductivity.
- The concept of absolute zero is essential in understanding the behavior of gases and other systems at extremely low temperatures.
In conclusion, according to the kinetic theory of gases, the absolute zero temperature is attained when the kinetic energy of the gas particles is zero. This temperature represents the lowest possible temperature and has important implications in the study of gases and low-temperature phenomena.
A closed system is one in which- a)mass does not cross boundaries of the system, though energy may do so
- b)mass crosses the boundary but not the energy
- c)neither mass nor energy crosses the boundaries of the system
- d)both energy and mass cross the boundaries of the system
- e)thermodynamic reactions take place
Correct answer is option 'A'. Can you explain this answer?
A closed system is one in which
a)
mass does not cross boundaries of the system, though energy may do so
b)
mass crosses the boundary but not the energy
c)
neither mass nor energy crosses the boundaries of the system
d)
both energy and mass cross the boundaries of the system
e)
thermodynamic reactions take place
|
|
Aditya Deshmukh answered |
In nonrelativistic classical mechanics, a closed system is a physical system that doesn't exchange any matter with its surroundings, and isn't subject to any net force whose source is external to the system. A closed system in classical mechanics would be considered an isolated system in thermodynamics.
On weight basis, air contains following parts of oxygen- a)21
- b)23
- c)25
- d)73
- e)79
Correct answer is option 'B'. Can you explain this answer?
On weight basis, air contains following parts of oxygen
a)
21
b)
23
c)
25
d)
73
e)
79

|
Ishan Kapoor answered |
Explanation:
Air is a mixture of various gases including oxygen, nitrogen, carbon dioxide, and other trace gases. The percentage of each gas in the air varies depending on the location and altitude. The following explanations will help to understand why the correct answer is option 'B'.
Composition of Air:
- Nitrogen: 78%
- Oxygen: 21%
- Argon: 0.9%
- Carbon dioxide: 0.04%
- Neon, helium, methane, krypton, hydrogen, and xenon: less than 0.1%
Calculation:
- The molecular weight of air is 28.97 g/mol.
- The molecular weight of oxygen is 32 g/mol.
- Therefore, the percentage of oxygen in air can be calculated as follows:
Percentage of oxygen in air = (32/28.97) x 100
= 21%
Thus, air contains 21% oxygen by weight.
Conclusion:
Therefore, option 'B' is the correct answer, which states that on a weight basis, air contains 23 parts of oxygen.
Air is a mixture of various gases including oxygen, nitrogen, carbon dioxide, and other trace gases. The percentage of each gas in the air varies depending on the location and altitude. The following explanations will help to understand why the correct answer is option 'B'.
Composition of Air:
- Nitrogen: 78%
- Oxygen: 21%
- Argon: 0.9%
- Carbon dioxide: 0.04%
- Neon, helium, methane, krypton, hydrogen, and xenon: less than 0.1%
Calculation:
- The molecular weight of air is 28.97 g/mol.
- The molecular weight of oxygen is 32 g/mol.
- Therefore, the percentage of oxygen in air can be calculated as follows:
Percentage of oxygen in air = (32/28.97) x 100
= 21%
Thus, air contains 21% oxygen by weight.
Conclusion:
Therefore, option 'B' is the correct answer, which states that on a weight basis, air contains 23 parts of oxygen.
If the value of n is high in the polytropic process, then the compressor work between given pressure limits will be- a)less
- b)more
- c)no effect
- d)zero
- e)infinite.
Correct answer is option 'A'. Can you explain this answer?
If the value of n is high in the polytropic process, then the compressor work between given pressure limits will be
a)
less
b)
more
c)
no effect
d)
zero
e)
infinite.
|
|
Sanya Agarwal answered |
-A polytropic process is a thermodynamic process that obeys the relation: {\displaystyle pV^{\, n}=C} where p is the pressure, V is volume, n is the polytropic index, and C is a constant. The polytropic process equation can describe multiple expansion and compression processes which include heat transfer.
-
If the value of n is high in the polytropic process, then the compressor work between given pressure limits will be
less.-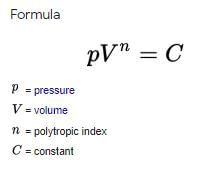
The unit of power in S.I. units is- a)newton
- b)pascal
- c)erg
- d)watt
- e)joule
Correct answer is option 'D'. Can you explain this answer?
The unit of power in S.I. units is
a)
newton
b)
pascal
c)
erg
d)
watt
e)
joule

|
Jaideep Malik answered |
Power is measured in Watts.
The specific heat of air increases with increase in- a)temperature
- b)pressure
- c)both pressure and temperature
- d)variation of its constituents
- e)air flow
Correct answer is option 'A'. Can you explain this answer?
The specific heat of air increases with increase in
a)
temperature
b)
pressure
c)
both pressure and temperature
d)
variation of its constituents
e)
air flow
|
|
Sarita Yadav answered |
- ANSWER a)temperature
The specific heat capacity of air increases with increase in temperature
because of the activation of the rotational and vibrational modes of energy at higher temperatures (as air is primarily composed of nitrogen and oxygen); so more energy is needed to increase the temperature of a particular mass of air.
According to Avogadro's Hypothesis- a)the molecular weights of all the perfect gases occupy the same volume under same conditions of pressure and temperature
- b)the sum of partial pressure of mixture of two gases is sum of the two
- c)product of the gas constant and the molecular weight of an ideal gas is constant
- d)gases have two values of specific heat
- e)all systems can be regarded as closed systems
Correct answer is option 'A'. Can you explain this answer?
According to Avogadro's Hypothesis
a)
the molecular weights of all the perfect gases occupy the same volume under same conditions of pressure and temperature
b)
the sum of partial pressure of mixture of two gases is sum of the two
c)
product of the gas constant and the molecular weight of an ideal gas is constant
d)
gases have two values of specific heat
e)
all systems can be regarded as closed systems

|
Anirban Khanna answered |
Avogadro's law states that, "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules." For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
Which of the following is the property of a system- a)pressure and temperature
- b)internal energy
- c)volume and density
- d)enthalpy and entropy
- e)all of the above
Correct answer is option 'E'. Can you explain this answer?
Which of the following is the property of a system
a)
pressure and temperature
b)
internal energy
c)
volume and density
d)
enthalpy and entropy
e)
all of the above
|
|
Kalyan Chakraborty answered |
Understanding the Properties of a System
In thermodynamics, a system is defined as a quantity of matter or a region in space chosen for analysis. The properties of a system are essential for understanding its behavior and characteristics. The options provided—pressure and temperature, internal energy, volume and density, enthalpy and entropy—each represent fundamental properties.
Key Properties Explained
Conclusion
Each of these properties plays a pivotal role in thermodynamic analysis. They are interrelated and collectively describe the state and behavior of a system under various conditions. Therefore, the correct answer is option 'E'—all of the above options are properties of a system, highlighting the comprehensive nature of thermodynamic study in mechanical engineering.
In thermodynamics, a system is defined as a quantity of matter or a region in space chosen for analysis. The properties of a system are essential for understanding its behavior and characteristics. The options provided—pressure and temperature, internal energy, volume and density, enthalpy and entropy—each represent fundamental properties.
Key Properties Explained
- Pressure and Temperature:
- Pressure is a measure of force applied per unit area, while temperature indicates the thermal state of a system. Both are crucial for defining the state of a system in thermodynamics. - Internal Energy:
- This is the total energy contained within a system, encompassing kinetic and potential energies of the molecules. It is vital for energy transfer calculations. - Volume and Density:
- Volume is the three-dimensional space occupied by a system, while density is mass per unit volume. These properties are essential for characterizing substances and their reactions. - Enthalpy and Entropy:
- Enthalpy is a measure of total energy (internal energy plus the product of pressure and volume), which is useful in thermodynamic processes. Entropy quantifies the degree of disorder in a system, reflecting the direction of spontaneous processes.
Conclusion
Each of these properties plays a pivotal role in thermodynamic analysis. They are interrelated and collectively describe the state and behavior of a system under various conditions. Therefore, the correct answer is option 'E'—all of the above options are properties of a system, highlighting the comprehensive nature of thermodynamic study in mechanical engineering.
Properties of substances like pressure, temperature and density, in thermodynamic coordinates are- a)path functions
- b)point functions
- c)cyclic function
- d)real functions
- e)thermodynamic functions
Correct answer is option 'B'. Can you explain this answer?
Properties of substances like pressure, temperature and density, in thermodynamic coordinates are
a)
path functions
b)
point functions
c)
cyclic function
d)
real functions
e)
thermodynamic functions

|
Sakshi Basak answered |
**Properties of substances in thermodynamic coordinates**
Properties of substances in thermodynamic coordinates, such as pressure, temperature, and density, can be classified as either path functions or point functions. In this case, the correct answer is option 'B' - point functions.
**Path functions**
Path functions are properties that depend on the path taken to reach a particular state or condition. These properties are typically associated with the transfer of energy or work. Examples of path functions in thermodynamics include heat transfer and work done on or by a system.
For example, the amount of heat transferred to a substance during a heating process depends on the specific path taken. Similarly, the work done on a gas during compression depends on the specific steps taken to reach the final state. Path functions are not solely determined by the initial and final states of a system but also depend on the process or path followed.
**Point functions**
In contrast, point functions are properties that depend only on the current state or condition of a system and are independent of the path taken to reach that state. These properties have a unique value for a given state and are not affected by the history of the system. Pressure, temperature, and density are examples of point functions.
Regardless of the path or process used to achieve a specific pressure, temperature, or density, these properties will have the same value if the system is in the same state. For instance, if a gas is at a pressure of 1 bar, it does not matter how the gas was brought to that pressure; the value of pressure will be the same. Similarly, the temperature of a substance will remain constant as long as the state of the substance remains unchanged, regardless of the path taken to reach that state.
**Conclusion**
In summary, properties of substances such as pressure, temperature, and density in thermodynamic coordinates are considered point functions. These properties depend only on the current state of a system and are independent of the path taken to reach that state.
Properties of substances in thermodynamic coordinates, such as pressure, temperature, and density, can be classified as either path functions or point functions. In this case, the correct answer is option 'B' - point functions.
**Path functions**
Path functions are properties that depend on the path taken to reach a particular state or condition. These properties are typically associated with the transfer of energy or work. Examples of path functions in thermodynamics include heat transfer and work done on or by a system.
For example, the amount of heat transferred to a substance during a heating process depends on the specific path taken. Similarly, the work done on a gas during compression depends on the specific steps taken to reach the final state. Path functions are not solely determined by the initial and final states of a system but also depend on the process or path followed.
**Point functions**
In contrast, point functions are properties that depend only on the current state or condition of a system and are independent of the path taken to reach that state. These properties have a unique value for a given state and are not affected by the history of the system. Pressure, temperature, and density are examples of point functions.
Regardless of the path or process used to achieve a specific pressure, temperature, or density, these properties will have the same value if the system is in the same state. For instance, if a gas is at a pressure of 1 bar, it does not matter how the gas was brought to that pressure; the value of pressure will be the same. Similarly, the temperature of a substance will remain constant as long as the state of the substance remains unchanged, regardless of the path taken to reach that state.
**Conclusion**
In summary, properties of substances such as pressure, temperature, and density in thermodynamic coordinates are considered point functions. These properties depend only on the current state of a system and are independent of the path taken to reach that state.
When a liquid boils at constant pressure, the following parameter increases- a)temperature
- b)heat of vaporisation
- c)kinetic energy
- d)entropy
- e)free energy
Correct answer is option 'D'. Can you explain this answer?
When a liquid boils at constant pressure, the following parameter increases
a)
temperature
b)
heat of vaporisation
c)
kinetic energy
d)
entropy
e)
free energy
|
|
Raj Kumar answered |
Boiling at Constant Pressure
Boiling is a process in which a liquid changes to a gas at a constant pressure. When a liquid boils at constant pressure, it absorbs heat from the surroundings to overcome the intermolecular forces between the molecules.
Increased Entropy
The correct answer to the question is option 'D', entropy. When a liquid boils at constant pressure, the entropy of the system increases. Entropy is a measure of the disorder or randomness of a system. When a liquid boils, the molecules gain enough energy to overcome the intermolecular forces holding them together, and they escape into the gas phase. This results in an increase in the randomness of the system, which is reflected in an increase in entropy.
Other Parameters
While temperature, heat of vaporisation, and kinetic energy all play a role in the process of boiling, they do not increase when a liquid boils at constant pressure.
- Temperature: The temperature of a liquid remains constant during the boiling process until all of the liquid has been converted to a gas. This is because the energy being added to the system is being used to overcome the intermolecular forces between the molecules, rather than to increase the temperature of the liquid.
- Heat of Vaporisation: The heat of vaporisation is the amount of energy required to convert a given amount of a liquid into a gas at a constant temperature. While the heat of vaporisation is an important parameter in the process of boiling, it does not change when a liquid boils at constant pressure.
- Kinetic Energy: The kinetic energy of the molecules in a liquid increases as the temperature of the liquid increases. However, when a liquid boils at constant pressure, the temperature remains constant, so the kinetic energy of the molecules does not increase.
Conclusion
In conclusion, when a liquid boils at constant pressure, the entropy of the system increases. This is because the boiling process results in an increase in the randomness of the system, which is reflected in an increase in entropy. While temperature, heat of vaporisation, and kinetic energy are all important parameters in the process of boiling, they do not increase when a liquid boils at constant pressure.
Boiling is a process in which a liquid changes to a gas at a constant pressure. When a liquid boils at constant pressure, it absorbs heat from the surroundings to overcome the intermolecular forces between the molecules.
Increased Entropy
The correct answer to the question is option 'D', entropy. When a liquid boils at constant pressure, the entropy of the system increases. Entropy is a measure of the disorder or randomness of a system. When a liquid boils, the molecules gain enough energy to overcome the intermolecular forces holding them together, and they escape into the gas phase. This results in an increase in the randomness of the system, which is reflected in an increase in entropy.
Other Parameters
While temperature, heat of vaporisation, and kinetic energy all play a role in the process of boiling, they do not increase when a liquid boils at constant pressure.
- Temperature: The temperature of a liquid remains constant during the boiling process until all of the liquid has been converted to a gas. This is because the energy being added to the system is being used to overcome the intermolecular forces between the molecules, rather than to increase the temperature of the liquid.
- Heat of Vaporisation: The heat of vaporisation is the amount of energy required to convert a given amount of a liquid into a gas at a constant temperature. While the heat of vaporisation is an important parameter in the process of boiling, it does not change when a liquid boils at constant pressure.
- Kinetic Energy: The kinetic energy of the molecules in a liquid increases as the temperature of the liquid increases. However, when a liquid boils at constant pressure, the temperature remains constant, so the kinetic energy of the molecules does not increase.
Conclusion
In conclusion, when a liquid boils at constant pressure, the entropy of the system increases. This is because the boiling process results in an increase in the randomness of the system, which is reflected in an increase in entropy. While temperature, heat of vaporisation, and kinetic energy are all important parameters in the process of boiling, they do not increase when a liquid boils at constant pressure.
Mixture of ice and water form a- a)closed system
- b)open system
- c)isolated system
- d)heterogeneous system
- e)thermodynamic system
Correct answer is option 'D'. Can you explain this answer?
Mixture of ice and water form a
a)
closed system
b)
open system
c)
isolated system
d)
heterogeneous system
e)
thermodynamic system
|
|
Devansh Nambiar answered |
Heterogeneous System:
A heterogeneous system is a system that consists of two or more phases or components that are physically distinct and identifiable.
In the case of a mixture of ice and water, it is a heterogeneous system because it consists of two phases - solid ice and liquid water. The ice and water can be physically separated and are distinguishable from each other.
Closed, Open, and Isolated Systems:
These terms refer to the type of system based on the exchange of matter and energy with the surroundings.
- A closed system is one that allows the transfer of energy but not matter with the surroundings.
- An open system is one that allows the transfer of both energy and matter with the surroundings.
- An isolated system is one that does not allow the transfer of either energy or matter with the surroundings.
In the case of a mixture of ice and water, none of these terms accurately describe the system as there is a transfer of matter (ice can melt into water) and energy (heat can be transferred) between the system and surroundings.
Therefore, the correct answer is that the mixture of ice and water forms a heterogeneous system.
A heterogeneous system is a system that consists of two or more phases or components that are physically distinct and identifiable.
In the case of a mixture of ice and water, it is a heterogeneous system because it consists of two phases - solid ice and liquid water. The ice and water can be physically separated and are distinguishable from each other.
Closed, Open, and Isolated Systems:
These terms refer to the type of system based on the exchange of matter and energy with the surroundings.
- A closed system is one that allows the transfer of energy but not matter with the surroundings.
- An open system is one that allows the transfer of both energy and matter with the surroundings.
- An isolated system is one that does not allow the transfer of either energy or matter with the surroundings.
In the case of a mixture of ice and water, none of these terms accurately describe the system as there is a transfer of matter (ice can melt into water) and energy (heat can be transferred) between the system and surroundings.
Therefore, the correct answer is that the mixture of ice and water forms a heterogeneous system.
Work done is zero for the following process- a)constant volume
- b)free expansion
- c)throttling
- d)all of the above
- e)none of the above
Correct answer is option 'D'. Can you explain this answer?
Work done is zero for the following process
a)
constant volume
b)
free expansion
c)
throttling
d)
all of the above
e)
none of the above

|
Rutuja Deshpande answered |
Work done is the energy transferred to or from a system by means of mechanical work. It is the product of the force applied to an object and the displacement of that object in the direction of the force.
a) Constant Volume Process:
In a constant volume process, the volume of the system remains constant. This means that there is no change in the displacement of the system. Since work done is the product of force and displacement, and there is no displacement in a constant volume process, the work done is zero.
b) Free Expansion Process:
In a free expansion process, a gas expands into a vacuum without any external force or resistance. Since there is no external force acting on the system, no work is done. The gas expands freely, and there is no displacement in the direction of the force. Therefore, the work done is zero.
c) Throttling Process:
Throttling is a process in which a fluid passes through a restriction, such as a valve or an orifice. In this process, the pressure of the fluid decreases while the enthalpy remains constant. Since there is no change in volume or displacement, no work is done during throttling. The work done is zero.
d) All of the Above:
From the explanations above, we can see that in a constant volume process, free expansion process, and throttling process, the work done is zero. Therefore, the correct answer is option 'D' - all of the above.
In summary, work done is zero in a constant volume process, free expansion process, and throttling process because there is no change in displacement or volume, and no external force is acting on the system.
a) Constant Volume Process:
In a constant volume process, the volume of the system remains constant. This means that there is no change in the displacement of the system. Since work done is the product of force and displacement, and there is no displacement in a constant volume process, the work done is zero.
b) Free Expansion Process:
In a free expansion process, a gas expands into a vacuum without any external force or resistance. Since there is no external force acting on the system, no work is done. The gas expands freely, and there is no displacement in the direction of the force. Therefore, the work done is zero.
c) Throttling Process:
Throttling is a process in which a fluid passes through a restriction, such as a valve or an orifice. In this process, the pressure of the fluid decreases while the enthalpy remains constant. Since there is no change in volume or displacement, no work is done during throttling. The work done is zero.
d) All of the Above:
From the explanations above, we can see that in a constant volume process, free expansion process, and throttling process, the work done is zero. Therefore, the correct answer is option 'D' - all of the above.
In summary, work done is zero in a constant volume process, free expansion process, and throttling process because there is no change in displacement or volume, and no external force is acting on the system.
Work done in an adiabatic process between a given pair of end state depends on- a)particular adiabatic process
- b)the end states only
- c)the value of index n
- d)the value of heat transferred
- e)mass of the system
Correct answer is option 'B'. Can you explain this answer?
Work done in an adiabatic process between a given pair of end state depends on
a)
particular adiabatic process
b)
the end states only
c)
the value of index n
d)
the value of heat transferred
e)
mass of the system
|
|
Mahi Kaur answered |
Explanation:
Adiabatic Process:
An adiabatic process is a thermodynamic process in which there is no heat transfer to or from the system. The word "adiabatic" comes from the Greek root "adiabatos," which means "not to be passed through." In an adiabatic process, the system is thermally isolated, so there is no heat transfer between the system and its surroundings. The adiabatic process can be reversible or irreversible, depending on whether the system can return to its initial state after the process.
Work Done in an Adiabatic Process:
The work done in an adiabatic process between a given pair of end states depends on several factors. However, the correct option among the given ones is option 'B,' which states that the work done depends only on the end states.
Factors Affecting Work Done in an Adiabatic Process:
There are several factors that affect the work done in an adiabatic process. Some of the significant ones are as follows:
Particular Adiabatic Process:
In an adiabatic process, the work done depends on the particular process. The process can be reversible or irreversible, and the work done will be different for each process.
Value of Index n:
The work done in an adiabatic process also depends on the value of the index n, which is the ratio of the specific heats of the gas. The value of n determines the relationship between pressure and volume during the process.
Value of Heat Transferred:
Since an adiabatic process is thermally isolated, there is no heat transfer between the system and its surroundings. Therefore, the work done in an adiabatic process does not depend on the value of heat transferred.
Mass of the System:
The work done in an adiabatic process does not depend on the mass of the system. The work done is a function of the pressure and volume of the system.
Conclusion:
In conclusion, the work done in an adiabatic process between a given pair of end states depends only on the end states and not on the other factors mentioned above. Therefore, option 'B' is the correct answer.
Adiabatic Process:
An adiabatic process is a thermodynamic process in which there is no heat transfer to or from the system. The word "adiabatic" comes from the Greek root "adiabatos," which means "not to be passed through." In an adiabatic process, the system is thermally isolated, so there is no heat transfer between the system and its surroundings. The adiabatic process can be reversible or irreversible, depending on whether the system can return to its initial state after the process.
Work Done in an Adiabatic Process:
The work done in an adiabatic process between a given pair of end states depends on several factors. However, the correct option among the given ones is option 'B,' which states that the work done depends only on the end states.
Factors Affecting Work Done in an Adiabatic Process:
There are several factors that affect the work done in an adiabatic process. Some of the significant ones are as follows:
Particular Adiabatic Process:
In an adiabatic process, the work done depends on the particular process. The process can be reversible or irreversible, and the work done will be different for each process.
Value of Index n:
The work done in an adiabatic process also depends on the value of the index n, which is the ratio of the specific heats of the gas. The value of n determines the relationship between pressure and volume during the process.
Value of Heat Transferred:
Since an adiabatic process is thermally isolated, there is no heat transfer between the system and its surroundings. Therefore, the work done in an adiabatic process does not depend on the value of heat transferred.
Mass of the System:
The work done in an adiabatic process does not depend on the mass of the system. The work done is a function of the pressure and volume of the system.
Conclusion:
In conclusion, the work done in an adiabatic process between a given pair of end states depends only on the end states and not on the other factors mentioned above. Therefore, option 'B' is the correct answer.
Which is true for reversible polytropic process- a)temperature remains constant
- b)entropy remains constant
- c)internal energy remains constant
- d)some heat transfer takes place.
- e)none of the above
Correct answer is option 'D'. Can you explain this answer?
Which is true for reversible polytropic process
a)
temperature remains constant
b)
entropy remains constant
c)
internal energy remains constant
d)
some heat transfer takes place.
e)
none of the above
|
|
Ishaan Malik answered |
Reversible Polytropic Process
A reversible polytropic process is a thermodynamic process in which the pressure and volume of a gas change while the temperature remains constant. It is a combination of two types of processes: isothermal and adiabatic. In an isothermal process, the temperature of the gas remains constant, while in an adiabatic process, there is no heat transfer between the gas and its surroundings. The polytropic process is reversible, meaning that it can be reversed without any loss of energy.
Option D is Correct
Option D, "some heat transfer takes place," is the correct answer for a reversible polytropic process. This is because a polytropic process is not isothermal or adiabatic, and therefore, there will be some heat transfer between the gas and its surroundings.
Explanation
During a reversible polytropic process, the gas undergoes a change in pressure and volume, which causes a change in its internal energy. This change in internal energy can be represented by the polytropic equation:
P V^n = C
where P is the pressure, V is the volume, n is the polytropic index, and C is a constant.
The polytropic index, n, depends on the specific process being studied. For example, if the process is isothermal, n = 1. If the process is adiabatic, n = γ, where γ is the ratio of specific heats.
In a reversible polytropic process, the gas undergoes a change in pressure and volume while the temperature remains constant. This means that the gas is not isothermal or adiabatic, and therefore, there will be some heat transfer between the gas and its surroundings.
Conclusion
In conclusion, a reversible polytropic process is a thermodynamic process in which the pressure and volume of a gas change while the temperature remains constant. The correct answer for a reversible polytropic process is option D, "some heat transfer takes place," as this process is not isothermal or adiabatic, and therefore, there will be some heat transfer between the gas and its surroundings.
A reversible polytropic process is a thermodynamic process in which the pressure and volume of a gas change while the temperature remains constant. It is a combination of two types of processes: isothermal and adiabatic. In an isothermal process, the temperature of the gas remains constant, while in an adiabatic process, there is no heat transfer between the gas and its surroundings. The polytropic process is reversible, meaning that it can be reversed without any loss of energy.
Option D is Correct
Option D, "some heat transfer takes place," is the correct answer for a reversible polytropic process. This is because a polytropic process is not isothermal or adiabatic, and therefore, there will be some heat transfer between the gas and its surroundings.
Explanation
During a reversible polytropic process, the gas undergoes a change in pressure and volume, which causes a change in its internal energy. This change in internal energy can be represented by the polytropic equation:
P V^n = C
where P is the pressure, V is the volume, n is the polytropic index, and C is a constant.
The polytropic index, n, depends on the specific process being studied. For example, if the process is isothermal, n = 1. If the process is adiabatic, n = γ, where γ is the ratio of specific heats.
In a reversible polytropic process, the gas undergoes a change in pressure and volume while the temperature remains constant. This means that the gas is not isothermal or adiabatic, and therefore, there will be some heat transfer between the gas and its surroundings.
Conclusion
In conclusion, a reversible polytropic process is a thermodynamic process in which the pressure and volume of a gas change while the temperature remains constant. The correct answer for a reversible polytropic process is option D, "some heat transfer takes place," as this process is not isothermal or adiabatic, and therefore, there will be some heat transfer between the gas and its surroundings.
Which of the following is not the intensive property- a)pressure
- b)temperature
- c)density
- d)heat
- e)specific volume
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not the intensive property
a)
pressure
b)
temperature
c)
density
d)
heat
e)
specific volume

|
Kiran Gupta answered |
Not all physical properties of a substance are the same. Some properties depend on the amount or size of the substance, while others do not. Intensive properties are those that do not depend on the quantity or size of the substance. In other words, they remain the same regardless of the amount of the substance present.
The intensive properties are important because they provide valuable information about the nature and behavior of a substance. They can be used to identify and characterize different substances, as well as to study their physical and chemical properties.
The given options are pressure, temperature, density, heat, and specific volume. Let's analyze each option to determine if it is an intensive property or not.
1. Pressure:
Pressure is defined as the force exerted per unit area. It is the ratio of force to the area over which the force is applied. Pressure is an intensive property because it does not depend on the amount of the substance present. The pressure of a gas, for example, remains the same regardless of the volume or quantity of the gas.
2. Temperature:
Temperature is a measure of the average kinetic energy of the particles in a substance. It is an intensive property because it does not depend on the amount of the substance. The temperature of a substance remains the same regardless of the quantity or size of the sample.
3. Density:
Density is defined as the mass of a substance per unit volume. It is an intensive property because it is independent of the amount of the substance. The density of a substance remains the same regardless of the quantity or size of the sample.
4. Heat:
Heat is the transfer of energy from a hotter object to a colder object. It is not an intensive property. The amount of heat transferred depends on the quantity of the substance. For example, if you have two objects of the same material at different temperatures, the amount of heat transferred will depend on the mass of the objects.
5. Specific volume:
Specific volume is defined as the volume occupied by a unit mass of a substance. It is an intensive property because it does not depend on the amount of the substance. The specific volume of a substance remains the same regardless of the quantity or size of the sample.
Therefore, the correct answer is option D, heat, as it is not an intensive property.
The intensive properties are important because they provide valuable information about the nature and behavior of a substance. They can be used to identify and characterize different substances, as well as to study their physical and chemical properties.
The given options are pressure, temperature, density, heat, and specific volume. Let's analyze each option to determine if it is an intensive property or not.
1. Pressure:
Pressure is defined as the force exerted per unit area. It is the ratio of force to the area over which the force is applied. Pressure is an intensive property because it does not depend on the amount of the substance present. The pressure of a gas, for example, remains the same regardless of the volume or quantity of the gas.
2. Temperature:
Temperature is a measure of the average kinetic energy of the particles in a substance. It is an intensive property because it does not depend on the amount of the substance. The temperature of a substance remains the same regardless of the quantity or size of the sample.
3. Density:
Density is defined as the mass of a substance per unit volume. It is an intensive property because it is independent of the amount of the substance. The density of a substance remains the same regardless of the quantity or size of the sample.
4. Heat:
Heat is the transfer of energy from a hotter object to a colder object. It is not an intensive property. The amount of heat transferred depends on the quantity of the substance. For example, if you have two objects of the same material at different temperatures, the amount of heat transferred will depend on the mass of the objects.
5. Specific volume:
Specific volume is defined as the volume occupied by a unit mass of a substance. It is an intensive property because it does not depend on the amount of the substance. The specific volume of a substance remains the same regardless of the quantity or size of the sample.
Therefore, the correct answer is option D, heat, as it is not an intensive property.
The molecular weight expressed in gm (i.e., 1 gm mole) of all gases at N.T.P. occupies a volume of- a)22.4 litres
- b)9.27 litres
- c)427 litres
- d)8.48 litres
- e)1 litres
Correct answer is option 'A'. Can you explain this answer?
The molecular weight expressed in gm (i.e., 1 gm mole) of all gases at N.T.P. occupies a volume of
a)
22.4 litres
b)
9.27 litres
c)
427 litres
d)
8.48 litres
e)
1 litres
|
|
Samarth Chaudhary answered |
Gas Laws and Avogadro's Law
The ideal gas law states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. At standard temperature and pressure (N.T.P.), the temperature is 273 K and the pressure is 1 atm.
Avogadro's law states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. This means that the volume occupied by one mole of any gas at N.T.P. is the same, regardless of the molecular weight of the gas.
Answer
Therefore, the correct answer is option A, which states that one mole of any gas at N.T.P. occupies a volume of 22.4 liters.
The ideal gas law states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. At standard temperature and pressure (N.T.P.), the temperature is 273 K and the pressure is 1 atm.
Avogadro's law states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. This means that the volume occupied by one mole of any gas at N.T.P. is the same, regardless of the molecular weight of the gas.
Answer
Therefore, the correct answer is option A, which states that one mole of any gas at N.T.P. occupies a volume of 22.4 liters.
The condition of perfect vacuum, i.e., absolute zero pressure can be attained at- a)a temperature of - 273.16°C
- b)a temperature of 0°C
- c)a temperature of 273°K
- d)a negative pressure and 0°C temperature
- e)can't be attained
Correct answer is option 'A'. Can you explain this answer?
The condition of perfect vacuum, i.e., absolute zero pressure can be attained at
a)
a temperature of - 273.16°C
b)
a temperature of 0°C
c)
a temperature of 273°K
d)
a negative pressure and 0°C temperature
e)
can't be attained
|
|
Ruchi Ahuja answered |
The condition of perfect vacuum, i.e., absolute zero pressure can be attained at. a temperature of - 273.16°C. a temperature of 0°C.
Which of the following quantities is not the property of the system- a)pressure
- b)temperature
- c)specific volume
- d)heat
- e)density
Correct answer is option 'D'. Can you explain this answer?
Which of the following quantities is not the property of the system
a)
pressure
b)
temperature
c)
specific volume
d)
heat
e)
density
|
|
Tarun Chatterjee answered |
Understanding System Properties
In thermodynamics, properties of a system describe its state and can be classified into two categories: state properties and path properties.
State Properties
State properties are characteristics that depend only on the state of the system and not on how that state was reached. The following are examples of state properties:
- Pressure: The force exerted per unit area within the system.
- Temperature: A measure of the average kinetic energy of the particles in a substance.
- Specific Volume: The volume occupied by a unit mass of a substance.
- Density: The mass per unit volume of a substance.
All these properties define the thermodynamic state of a system and are independent of the processes that lead to that state.
Path Properties
In contrast, heat is a path property.
- Heat: It represents energy transfer due to temperature difference and is dependent on the specific path or process taken to change the state of the system.
Conclusion
Thus, the correct answer to the question is:
- Option D: Heat is not a property of the system. It is a form of energy transfer that depends on how the system transitions between states rather than the state itself.
Understanding the distinction between state and path properties is crucial in thermodynamics for analyzing and modeling physical systems effectively.
In thermodynamics, properties of a system describe its state and can be classified into two categories: state properties and path properties.
State Properties
State properties are characteristics that depend only on the state of the system and not on how that state was reached. The following are examples of state properties:
- Pressure: The force exerted per unit area within the system.
- Temperature: A measure of the average kinetic energy of the particles in a substance.
- Specific Volume: The volume occupied by a unit mass of a substance.
- Density: The mass per unit volume of a substance.
All these properties define the thermodynamic state of a system and are independent of the processes that lead to that state.
Path Properties
In contrast, heat is a path property.
- Heat: It represents energy transfer due to temperature difference and is dependent on the specific path or process taken to change the state of the system.
Conclusion
Thus, the correct answer to the question is:
- Option D: Heat is not a property of the system. It is a form of energy transfer that depends on how the system transitions between states rather than the state itself.
Understanding the distinction between state and path properties is crucial in thermodynamics for analyzing and modeling physical systems effectively.
Which of the following laws is applicable for the behaviour of a perfect gas- a)Boyle's law
- b)Charle's law
- c)Gay-Lussac law
- d)all of the above
- e)Joule's law
Correct answer is option 'D'. Can you explain this answer?
Which of the following laws is applicable for the behaviour of a perfect gas
a)
Boyle's law
b)
Charle's law
c)
Gay-Lussac law
d)
all of the above
e)
Joule's law

|
Kaavya Sengupta answered |
Joule’s law, in electricity, mathematical description of the rate at which resistance in a circuit converts electric energy into heat energy. The English physicist James Prescott Joule discovered in 1840 that the amount of heat per second that develops in a wire carrying a current is proportional to the electrical resistance of the wire and the square of the current. He determined that the heat evolved per second is equivalent to the electric power absorbed, or the power loss.
A quantitative form of Joule’s law is that the heat evolved per second, or the electric power loss, P, equals the current I squared times the resistance R, or P = I2R. The power P has units of watts, or joules per second, when the current is expressed in amperes and the resistance in ohms.
Chapter doubts & questions for Thermodynamics - Mechanical Engineering SSC JE (Technical) 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermodynamics - Mechanical Engineering SSC JE (Technical) in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Mechanical Engineering SSC JE (Technical)
6 videos|104 docs|59 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup