All Exams >
Chemistry >
Physical Chemistry >
All Questions
All questions of Atomic and Molecular Structure for Chemistry Exam
Can you explain the answer of this question below:The threshold frequency v0 for a metal is 7.0 x 1014 s-1. Radiation of frequency v = 1.0 x 1015 s-1 hits the metal. Kinetic energy of the emitted electron is
- A:
2.0 x 10-18 J
- B:
1.60 x 10-17 J
- C:
1.60 x 10-19 J
- D:
2.0 x 10-19 J
The answer is d.
The threshold frequency v0 for a metal is 7.0 x 1014 s-1. Radiation of frequency v = 1.0 x 1015 s-1 hits the metal. Kinetic energy of the emitted electron is
2.0 x 10-18 J
1.60 x 10-17 J
1.60 x 10-19 J
2.0 x 10-19 J
|
|
Suresh Reddy answered |
By photoelectric effect
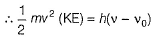
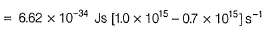
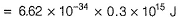
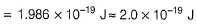
Direction (Q. Nos. 11 and 12) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)Photoelectric effect can be expressed in terms of the following graph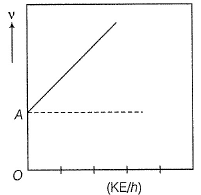
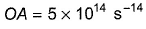 Q. What is work function for this photoelectric emission of electrons?
Q. What is work function for this photoelectric emission of electrons?- a)199.262 kJ mol-1
- b)199.262 J mol-1
- c)3.3 kJ mol-1
- d)3.3 x 10-19 kJ mol-1
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 11 and 12) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)
Photoelectric effect can be expressed in terms of the following graph
Q. What is work function for this photoelectric emission of electrons?
a)
199.262 kJ mol-1
b)
199.262 J mol-1
c)
3.3 kJ mol-1
d)
3.3 x 10-19 kJ mol-1
|
|
Gaurav Kumar answered |
Photoelectric effect is represented by
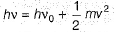
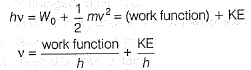

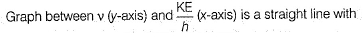
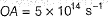
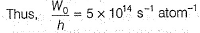
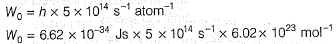
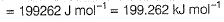
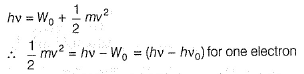
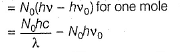
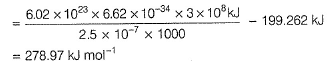
Which of the following can be a solution of Schrodinger equation?- a)
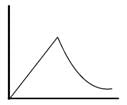
- b)
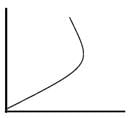
- c)
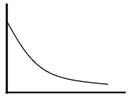
- d)
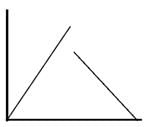
Correct answer is option 'C'. Can you explain this answer?
Which of the following can be a solution of Schrodinger equation?
a)

b)

c)

d)


|
Pioneer Academy answered |
Out of the following, only the below diagram can be the solution of the Schrodinger Wave equation. because other diagram does not have a continuous dΨ/dx. Some diagrams are double valued and discontinuous also.

The position of both, an electron and a helium atom is known within 1.0 mm. Further more the momentum of the electron is known within 5.0 x 10-26 kg ms-1. The minimum uncertainty in the measurement of the momentum of the helium atom is- a)50.0 kg ms-1
- b)80.0 kg ms-1
- c)80.0 x 10-26 kg ms-1
- d)5.0 x 10-26 kg ms-1
Correct answer is option 'D'. Can you explain this answer?
The position of both, an electron and a helium atom is known within 1.0 mm. Further more the momentum of the electron is known within 5.0 x 10-26 kg ms-1. The minimum uncertainty in the measurement of the momentum of the helium atom is
a)
50.0 kg ms-1
b)
80.0 kg ms-1
c)
80.0 x 10-26 kg ms-1
d)
5.0 x 10-26 kg ms-1

|
Nabanita Singh answered |
Given,
Position of both an electron and a Helium atom = 1 nm
The momentum of an electron =
Uncertainty principle: It is defined as the position and the momentum both can not be determined simultaneously.
According to the Uncertainty principle,

h = Planck`s constant
When the position of an electron and helium atom is the same and the momentum of an electron is known then the momentum of the helium atom is equal to the momentum of an electron.
Therefore, the momentum of the Helium atom is 5.0 x 10-26 kg ms-1
According to the Aufbau’s principle, which of the following orbital should be filled first?- a)5d
- b)4p
- c)3p
- d)2s
Correct answer is option 'D'. Can you explain this answer?
According to the Aufbau’s principle, which of the following orbital should be filled first?
a)
5d
b)
4p
c)
3p
d)
2s
|
|
Vivek Khatri answered |
As per the Aufbau’s principle, the orbital or subshell with the lowest energy should be filled first. The ascending order of orbital’s energy is given by 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 4f, 5d, 6p, 7s. So 2s orbital should be filled first.
Statement I : It is impossible to determine position and momentum of the moving electron simultaneously with same accuracy.Statement II : The path of an electron in an atom is clearly defined.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'C'. Can you explain this answer?
Statement I : It is impossible to determine position and momentum of the moving electron simultaneously with same accuracy.
Statement II : The path of an electron in an atom is clearly defined.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Neha Joshi answered |
Statement 1 is uncertainty principle, according to which we can't locate a moving electron accurately at any instant, which implies there must me certain uncertainty in its path, as path basically gives us the position of electron at various instances.
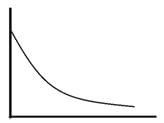
Find the function, f(x), for which X 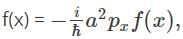 where a is the real quantity.
where a is the real quantity.- a)ke-x2
- b)ke-x2/2a
- c)ke-x2/2a2
- d)ke-x2/3a
Correct answer is option 'C'. Can you explain this answer?
Find the function, f(x), for which X 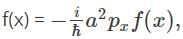 where a is the real quantity.
where a is the real quantity.
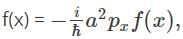 where a is the real quantity.
where a is the real quantity.a)
ke-x2
b)
ke-x2/2a
c)
ke-x2/2a2
d)
ke-x2/3a
|
|
Vivek Khatri answered |
Now, given that, X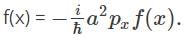
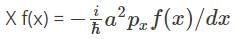
df/f = -xdx/a2
ln f = -x2/2a2 + C
f = ke-x2/2a2.
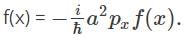
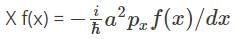
df/f = -xdx/a2
ln f = -x2/2a2 + C
f = ke-x2/2a2.
Photoelectric effect can be expressed in terms of the following graph
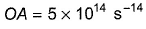 Q. Kinetic energy imparted to the moving electron at a wavelength of 2.5 x 10-7 m is
Q. Kinetic energy imparted to the moving electron at a wavelength of 2.5 x 10-7 m is- a)-278.97 kJ mol-1
- b)278.97 kJmol-1
- c)168.23 kJmol-1
- d)-168.23 kJ mol-1
Correct answer is option 'B'. Can you explain this answer?
Photoelectric effect can be expressed in terms of the following graph
Q. Kinetic energy imparted to the moving electron at a wavelength of 2.5 x 10-7 m is
a)
-278.97 kJ mol-1
b)
278.97 kJmol-1
c)
168.23 kJmol-1
d)
-168.23 kJ mol-1

|
Pioneer Academy answered |
Photoelectric effect is represented by
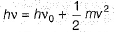


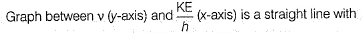
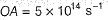
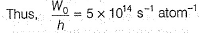
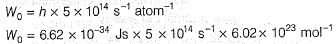
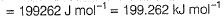
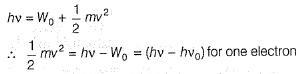
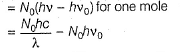
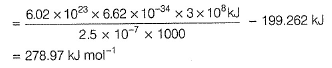
Iω = _________- a)mev2r
- b)mevr2
- c)mer
- d)mevr
Correct answer is option 'D'. Can you explain this answer?
Iω = _________
a)
mev2r
b)
mevr2
c)
mer
d)
mevr

|
Aryan Choudhary answered |
Explanation:
Angular Momentum:
- Angular momentum (I) is defined as the product of the moment of inertia (I) and the angular velocity (ω) of an object rotating around an axis.
- Mathematically, Iω = Iωr, where r is the radius of rotation.
Units of Angular Momentum:
- The units of angular momentum in the International System of Units (SI) are kilogram square meter per second (kg m²/s).
- In terms of base SI units, this can be written as kg * m * m/s.
Given Expression:
- Here, the expression Iω = mevr is provided.
- The correct unit for the angular momentum in this case would be kg * m * m/s * r, which corresponds to option 'D' (mevr).
Therefore, the correct answer is option 'D' - mevr.
Angular Momentum:
- Angular momentum (I) is defined as the product of the moment of inertia (I) and the angular velocity (ω) of an object rotating around an axis.
- Mathematically, Iω = Iωr, where r is the radius of rotation.
Units of Angular Momentum:
- The units of angular momentum in the International System of Units (SI) are kilogram square meter per second (kg m²/s).
- In terms of base SI units, this can be written as kg * m * m/s.
Given Expression:
- Here, the expression Iω = mevr is provided.
- The correct unit for the angular momentum in this case would be kg * m * m/s * r, which corresponds to option 'D' (mevr).
Therefore, the correct answer is option 'D' - mevr.
The energy of a hydrogen atom is positive.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
The energy of a hydrogen atom is positive.
a)
True
b)
False
|
|
Vivek Khatri answered |
The energy of a hydrogen atom is negative. It means the energy of a hydrogen atom is then that lower than that of a free electron that is at rest. This means the hydrogen atom has negative electronic energy.
Frequency of a matter wave is equal to- a)
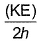
- b)
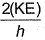
- c)
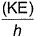
- d)
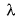
Correct answer is option 'B'. Can you explain this answer?
Frequency of a matter wave is equal to
a)
b)
c)
d)
|
|
Chirag Verma answered |
By de-Broglie equation
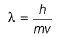
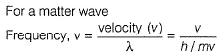
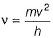
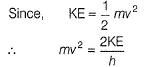
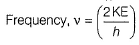
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 x 107 ms-1, then binding energy by which electron is bound to nucleus is- a)1.223 x10-15 J
- b)-1.223x 10-15 J
- c)2.345 x10-15 J
- d)1.428 x10-15 J
Correct answer is option 'B'. Can you explain this answer?
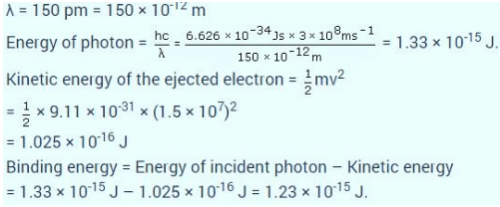
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 x 107 ms-1, then binding energy by which electron is bound to nucleus is
a)
1.223 x10-15 J
b)
-1.223x 10-15 J
c)
2.345 x10-15 J
d)
1.428 x10-15 J
|
|
Shreya Gupta answered |
Which of the following statements you think is wrong regarding α particle scattering effect?- a)α particles mostly move through the gold foil having zero deflection
- b)A small fraction are deflected
- c)One in Twenty Thousand turns 180°
- d)The thickness of the gold foil is about 100μm
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements you think is wrong regarding α particle scattering effect?
a)
α particles mostly move through the gold foil having zero deflection
b)
A small fraction are deflected
c)
One in Twenty Thousand turns 180°
d)
The thickness of the gold foil is about 100μm
|
|
Vivek Khatri answered |
In this effect, a thin foil (thickness 100nm) made up of gold and coated with fluorescent ZnS screen which is circular around it. α particles mostly move through the gold foil having zero deflection, a small fraction is deflected and one in twenty thousand turns 180°.
What is the shape the orbital, whose “l” is 1?- a)Spherical
- b)Dumbbell
- c)Double dumbbell
- d)Complex
Correct answer is option 'B'. Can you explain this answer?
What is the shape the orbital, whose “l” is 1?
a)
Spherical
b)
Dumbbell
c)
Double dumbbell
d)
Complex
|
|
Vivek Khatri answered |
The azimuthal quantum number is given by “l”. When l = 0, 1, 2 and 3, they are s-orbital, p-orbital, d-orbital and f-orbital respectively. The shapes of s-orbital, p-orbital, d-orbital, and f-orbital are Spherical, Dumbbell, Double dumbbell and Complex respectively.
Calculate the wavelength of a photon that traveled from 5th orbit to 2nd orbit.- a)434 nm
- b)456 nm
- c)863 nm
- d)268 nm
Correct answer is option 'A'. Can you explain this answer?
Calculate the wavelength of a photon that traveled from 5th orbit to 2nd orbit.
a)
434 nm
b)
456 nm
c)
863 nm
d)
268 nm
|
|
Vivek Khatri answered |
The energy of an nth orbit in a hydrogen atom is given by the formula En = -RH/n2, where is the energy of nth orbit and RH is the Rydberg constant. E5 – E2 = -4.58 x 10-19J. λ(wavelength) = c(speed of light)h(Planck’s constant)/E = 434nm.
Which of the following set of quantum numbers is not valid?- a)n = 5, l = 2, m = 0, s = 1/2
- b)n = 1, l = 2, m = 0, s = 1/2
- c)n = 5, l = 3, m = 2, s = 1/2
- d)n = 5, l = 2, m = 0, s = -1/2
Correct answer is option 'B'. Can you explain this answer?
Which of the following set of quantum numbers is not valid?
a)
n = 5, l = 2, m = 0, s = 1/2
b)
n = 1, l = 2, m = 0, s = 1/2
c)
n = 5, l = 3, m = 2, s = 1/2
d)
n = 5, l = 2, m = 0, s = -1/2

|
Kiran Pillai answered |
**Explanation:**
In quantum mechanics, each electron in an atom is described by a set of four quantum numbers: principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (m), and spin quantum number (s). These quantum numbers determine the energy, shape, orientation, and spin of an electron within an atom.
Let's analyze each option to determine if it is a valid set of quantum numbers:
**a) n = 5, l = 2, m = 0, s = 1/2**
- **n = 5**: This indicates the principal quantum number and represents the energy level of the electron. It can have any positive integer value.
- **l = 2**: This represents the azimuthal quantum number, which determines the shape of the electron's orbital. It can have values ranging from 0 to (n-1). Therefore, l = 2 is valid for n = 5.
- **m = 0**: This represents the magnetic quantum number and determines the orientation of the electron's orbital in space. It can have values ranging from -l to +l. Therefore, m = 0 is valid for l = 2.
- **s = 1/2**: This represents the spin quantum number, which indicates the spin state of the electron. It can have values of +1/2 or -1/2. Therefore, s = 1/2 is valid.
Since all the quantum numbers in option a) are valid, it is a valid set of quantum numbers.
**b) n = 1, l = 2, m = 0, s = 1/2**
- **n = 1**: This represents the principal quantum number and represents the energy level of the electron. It can have any positive integer value.
- **l = 2**: This represents the azimuthal quantum number, which determines the shape of the electron's orbital. It can have values ranging from 0 to (n-1). However, in this case, n = 1, so l cannot be greater than (n-1). Therefore, l = 2 is not valid for n = 1.
Since the value of l is not valid for the given value of n, option b) is not a valid set of quantum numbers.
**c) n = 5, l = 3, m = 2, s = 1/2**
- **n = 5**: This indicates the principal quantum number and represents the energy level of the electron. It can have any positive integer value.
- **l = 3**: This represents the azimuthal quantum number, which determines the shape of the electron's orbital. It can have values ranging from 0 to (n-1). Therefore, l = 3 is valid for n = 5.
- **m = 2**: This represents the magnetic quantum number and determines the orientation of the electron's orbital in space. It can have values ranging from -l to +l. Therefore, m = 2 is valid for l = 3.
- **s = 1/2**: This represents the spin quantum number, which indicates the spin state of the electron. It can have values of +1/2 or -1/2. Therefore, s = 1/2 is valid.
Since all the quantum numbers in option c) are valid, it is a
In quantum mechanics, each electron in an atom is described by a set of four quantum numbers: principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (m), and spin quantum number (s). These quantum numbers determine the energy, shape, orientation, and spin of an electron within an atom.
Let's analyze each option to determine if it is a valid set of quantum numbers:
**a) n = 5, l = 2, m = 0, s = 1/2**
- **n = 5**: This indicates the principal quantum number and represents the energy level of the electron. It can have any positive integer value.
- **l = 2**: This represents the azimuthal quantum number, which determines the shape of the electron's orbital. It can have values ranging from 0 to (n-1). Therefore, l = 2 is valid for n = 5.
- **m = 0**: This represents the magnetic quantum number and determines the orientation of the electron's orbital in space. It can have values ranging from -l to +l. Therefore, m = 0 is valid for l = 2.
- **s = 1/2**: This represents the spin quantum number, which indicates the spin state of the electron. It can have values of +1/2 or -1/2. Therefore, s = 1/2 is valid.
Since all the quantum numbers in option a) are valid, it is a valid set of quantum numbers.
**b) n = 1, l = 2, m = 0, s = 1/2**
- **n = 1**: This represents the principal quantum number and represents the energy level of the electron. It can have any positive integer value.
- **l = 2**: This represents the azimuthal quantum number, which determines the shape of the electron's orbital. It can have values ranging from 0 to (n-1). However, in this case, n = 1, so l cannot be greater than (n-1). Therefore, l = 2 is not valid for n = 1.
Since the value of l is not valid for the given value of n, option b) is not a valid set of quantum numbers.
**c) n = 5, l = 3, m = 2, s = 1/2**
- **n = 5**: This indicates the principal quantum number and represents the energy level of the electron. It can have any positive integer value.
- **l = 3**: This represents the azimuthal quantum number, which determines the shape of the electron's orbital. It can have values ranging from 0 to (n-1). Therefore, l = 3 is valid for n = 5.
- **m = 2**: This represents the magnetic quantum number and determines the orientation of the electron's orbital in space. It can have values ranging from -l to +l. Therefore, m = 2 is valid for l = 3.
- **s = 1/2**: This represents the spin quantum number, which indicates the spin state of the electron. It can have values of +1/2 or -1/2. Therefore, s = 1/2 is valid.
Since all the quantum numbers in option c) are valid, it is a
Which of the following can be a wave function?- a)tan x
- b)sin x
- c)cot x
- d)sec x
Correct answer is option 'B'. Can you explain this answer?
Which of the following can be a wave function?
a)
tan x
b)
sin x
c)
cot x
d)
sec x
|
|
Vivek Khatri answered |
Out of all the given options, sin x is the only function, that is continuous and single-valued. All the rest of the functions are either discontinuous or double-valued.
What is the absolute charge of a proton?
- a)+1.602176×10-27
- b)+1.602176×10-19
- c)–1.602176×10-19
- d)–1.602176×10-27
Correct answer is option 'B'. Can you explain this answer?
What is the absolute charge of a proton?
a)
+1.602176×10-27
b)
+1.602176×10-19
c)
–1.602176×10-19
d)
–1.602176×10-27
|
|
Vivek Khatri answered |
According to the fundamental properties of particles, protons charge is +1.602176×10-19C. It is a subatomic particle. Rutherford discovered protons. Its elementary charge is 1. Proton’s charge is positive.
How many spectral lines does hydrogen have?- a)Four
- b)Three
- c)Two
- d)One
Correct answer is option 'A'. Can you explain this answer?
How many spectral lines does hydrogen have?
a)
Four
b)
Three
c)
Two
d)
One

|
Arnab Pillai answered |
Hydrogen is a chemical element with atomic number 1. It is the lightest element and also the most abundant element in the universe. Hydrogen has a unique spectral signature that allows scientists to identify it in space and in the laboratory.
Spectral Lines of Hydrogen
When hydrogen is excited, it emits light at specific wavelengths. This light can be separated into its component colors using a prism or a diffraction grating. The resulting spectrum is a series of colored lines or bands that correspond to the different wavelengths of light emitted by the excited hydrogen atoms.
The spectral lines of hydrogen are classified into several series based on the energy transitions that produce them. These series are named after their discoverers and are designated by letters. The four main series of hydrogen spectral lines are:
1. Lyman series (n=1): This series consists of spectral lines that originate from the n=1 energy level of hydrogen. The Lyman series lines are in the ultraviolet region of the electromagnetic spectrum and are not visible to the naked eye.
2. Balmer series (n=2): This series consists of spectral lines that originate from the n=2 energy level of hydrogen. The Balmer series lines are in the visible region of the electromagnetic spectrum and are easily observable.
3. Paschen series (n=3): This series consists of spectral lines that originate from the n=3 energy level of hydrogen. The Paschen series lines are in the infrared region of the electromagnetic spectrum and are not visible to the naked eye.
4. Brackett series (n=4): This series consists of spectral lines that originate from the n=4 energy level of hydrogen. The Brackett series lines are in the infrared region of the electromagnetic spectrum and are not visible to the naked eye.
Conclusion
In summary, hydrogen has four spectral lines that correspond to the Lyman, Balmer, Paschen, and Brackett series. These spectral lines are important for identifying hydrogen in space and in the laboratory, and for studying the energy levels of hydrogen atoms.
Spectral Lines of Hydrogen
When hydrogen is excited, it emits light at specific wavelengths. This light can be separated into its component colors using a prism or a diffraction grating. The resulting spectrum is a series of colored lines or bands that correspond to the different wavelengths of light emitted by the excited hydrogen atoms.
The spectral lines of hydrogen are classified into several series based on the energy transitions that produce them. These series are named after their discoverers and are designated by letters. The four main series of hydrogen spectral lines are:
1. Lyman series (n=1): This series consists of spectral lines that originate from the n=1 energy level of hydrogen. The Lyman series lines are in the ultraviolet region of the electromagnetic spectrum and are not visible to the naked eye.
2. Balmer series (n=2): This series consists of spectral lines that originate from the n=2 energy level of hydrogen. The Balmer series lines are in the visible region of the electromagnetic spectrum and are easily observable.
3. Paschen series (n=3): This series consists of spectral lines that originate from the n=3 energy level of hydrogen. The Paschen series lines are in the infrared region of the electromagnetic spectrum and are not visible to the naked eye.
4. Brackett series (n=4): This series consists of spectral lines that originate from the n=4 energy level of hydrogen. The Brackett series lines are in the infrared region of the electromagnetic spectrum and are not visible to the naked eye.
Conclusion
In summary, hydrogen has four spectral lines that correspond to the Lyman, Balmer, Paschen, and Brackett series. These spectral lines are important for identifying hydrogen in space and in the laboratory, and for studying the energy levels of hydrogen atoms.
If the uncertainties in the measurements of position and momentum are equal, calculate the uncertainty in the measurement of velocity (in ms-1) of particle of mass 1.21 x 10-18 kg
Correct answer is '6'. Can you explain this answer?
If the uncertainties in the measurements of position and momentum are equal, calculate the uncertainty in the measurement of velocity (in ms-1) of particle of mass 1.21 x 10-18 kg

|
Arnav Kulkarni answered |
Uncertainty principle:
The uncertainty principle states that it is impossible to simultaneously measure the exact position and momentum of a particle with complete accuracy. The more precisely we try to measure one of these quantities, the less precisely we can know the other.
Given information:
- The uncertainties in the measurements of position and momentum are equal.
- The mass of the particle is 1.21 x 10^-18 kg.
Mathematical representation:
The uncertainty principle is mathematically represented as Δx * Δp ≥ h/(4π), where Δx is the uncertainty in position, Δp is the uncertainty in momentum, and h is the Planck's constant (6.626 x 10^-34 Js).
Equal uncertainties:
Since the uncertainties in position and momentum are equal, we can write Δx = Δp.
Calculating the uncertainty in velocity:
Velocity is defined as the rate of change of position with respect to time. It is given by the equation v = Δx/Δt, where v is velocity, Δx is the change in position, and Δt is the change in time.
We can rearrange this equation to express the change in position as Δx = v * Δt.
Substituting the value of Δx in the uncertainty principle equation, we get Δp * v * Δt ≥ h/(4π).
Since Δp = Δx, we can rewrite the equation as Δx * v * Δt ≥ h/(4π).
Simplifying further, we have v * Δt ≥ h/(4πΔx).
The uncertainty in velocity is given by Δv = v * Δt.
Substituting the value of Δt in the equation, we get Δv = v * (h/(4πΔx))/v.
Simplifying further, we have Δv = h/(4πΔx).
Substituting Δx = Δp, we have Δv = h/(4πΔp).
Substituting the value of h and Δp, we get Δv = (6.626 x 10^-34 Js)/(4πΔp).
Since Δp = Δx, we can write Δv = (6.626 x 10^-34 Js)/(4πΔx).
Given that Δx = Δp, we can further simplify the equation to Δv = (6.626 x 10^-34 Js)/(4πΔp) = (6.626 x 10^-34 Js)/(4πΔx).
Calculating the uncertainty in velocity:
Substituting the value of Δx = Δp = Δv, we get Δv = (6.626 x 10^-34 Js)/(4πΔv).
Simplifying further, we have Δv^2 = (6.626 x 10^-34 Js)/(4π).
Taking the square root of both sides, we have Δv = √[(6.626 x 10^-34 Js)/(4π)].
Calculating the numerical value, we find Δv ≈ 6 m/s.
Therefore, the uncertainty in the measurement of velocity is approximately 6 m/s.
The uncertainty principle states that it is impossible to simultaneously measure the exact position and momentum of a particle with complete accuracy. The more precisely we try to measure one of these quantities, the less precisely we can know the other.
Given information:
- The uncertainties in the measurements of position and momentum are equal.
- The mass of the particle is 1.21 x 10^-18 kg.
Mathematical representation:
The uncertainty principle is mathematically represented as Δx * Δp ≥ h/(4π), where Δx is the uncertainty in position, Δp is the uncertainty in momentum, and h is the Planck's constant (6.626 x 10^-34 Js).
Equal uncertainties:
Since the uncertainties in position and momentum are equal, we can write Δx = Δp.
Calculating the uncertainty in velocity:
Velocity is defined as the rate of change of position with respect to time. It is given by the equation v = Δx/Δt, where v is velocity, Δx is the change in position, and Δt is the change in time.
We can rearrange this equation to express the change in position as Δx = v * Δt.
Substituting the value of Δx in the uncertainty principle equation, we get Δp * v * Δt ≥ h/(4π).
Since Δp = Δx, we can rewrite the equation as Δx * v * Δt ≥ h/(4π).
Simplifying further, we have v * Δt ≥ h/(4πΔx).
The uncertainty in velocity is given by Δv = v * Δt.
Substituting the value of Δt in the equation, we get Δv = v * (h/(4πΔx))/v.
Simplifying further, we have Δv = h/(4πΔx).
Substituting Δx = Δp, we have Δv = h/(4πΔp).
Substituting the value of h and Δp, we get Δv = (6.626 x 10^-34 Js)/(4πΔp).
Since Δp = Δx, we can write Δv = (6.626 x 10^-34 Js)/(4πΔx).
Given that Δx = Δp, we can further simplify the equation to Δv = (6.626 x 10^-34 Js)/(4πΔp) = (6.626 x 10^-34 Js)/(4πΔx).
Calculating the uncertainty in velocity:
Substituting the value of Δx = Δp = Δv, we get Δv = (6.626 x 10^-34 Js)/(4πΔv).
Simplifying further, we have Δv^2 = (6.626 x 10^-34 Js)/(4π).
Taking the square root of both sides, we have Δv = √[(6.626 x 10^-34 Js)/(4π)].
Calculating the numerical value, we find Δv ≈ 6 m/s.
Therefore, the uncertainty in the measurement of velocity is approximately 6 m/s.
Which of the following rules/principles is responsible to rule out the existence of definite paths or trajectories of electrons?- a)Aufbau (ascending energy) rule
- b)Hund’s rule of maximum multiples
- c)Pauli’s exclusion principle
- d)Heisenberg’s uncertainty principle
Correct answer is option 'D'. Can you explain this answer?
Which of the following rules/principles is responsible to rule out the existence of definite paths or trajectories of electrons?
a)
Aufbau (ascending energy) rule
b)
Hund’s rule of maximum multiples
c)
Pauli’s exclusion principle
d)
Heisenberg’s uncertainty principle
|
|
Anjana Sharma answered |
According to Heisenberg’s uncertainty principle, the position and velocity of an electron cannot be determined simultaneously with accuracy which rules out the existence of fixed paths.
Total number of nodes for 3d orbital is ________- a)3
- b)2
- c)1
- d)0
Correct answer is option 'B'. Can you explain this answer?
Total number of nodes for 3d orbital is ________
a)
3
b)
2
c)
1
d)
0
|
|
Vivek Khatri answered |
Total number of nodes include angular and radial nodes. Angular nodes and radial nodes are given by the formula n – l -1 and l respectively. So the total number of nodes are n – l -1 + l = n – 1. For 3d orbit, “n” is 3, so total number nodes is 3 – 1 = 2.
Gravitational force = Gm1m2/r2.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
Gravitational force = Gm1m2/r2.
a)
True
b)
False

|
Niharika Kulkarni answered |
Gravitational Force Formula
The gravitational force between two objects is given by the equation:
F = G * (m1 * m2) / r^2
where F is the gravitational force, G is the gravitational constant, m1 and m2 are the masses of the two objects, and r is the distance between their centers of mass.
Explanation
The statement "Gravitational force = Gm1m2/r^2" is true because it represents the formula for calculating the gravitational force between two objects. Here is a detailed explanation of each component of the formula:
1. G: The gravitational constant is denoted by G and has a value of approximately 6.67430 × 10^-11 N(m/kg)^2. It is a fundamental constant in physics that determines the strength of the gravitational force.
2. m1 and m2: These variables represent the masses of the two objects involved in the gravitational interaction. The gravitational force is directly proportional to the product of their masses. As the masses increase, the gravitational force between the objects also increases.
3. r: The distance between the centers of mass of the two objects is denoted by r. The gravitational force is inversely proportional to the square of the distance between the objects. As the distance increases, the gravitational force decreases.
4. F: The gravitational force is the force of attraction between the two objects. It acts along the line connecting their centers of mass and is always an attractive force.
Conclusion
In summary, the equation "Gravitational force = Gm1m2/r^2" is true and represents the formula for calculating the gravitational force between two objects. It takes into account the gravitational constant, the masses of the objects, and the distance between them. By understanding this formula, we can quantitatively analyze and predict the gravitational interactions between various objects.
The gravitational force between two objects is given by the equation:
F = G * (m1 * m2) / r^2
where F is the gravitational force, G is the gravitational constant, m1 and m2 are the masses of the two objects, and r is the distance between their centers of mass.
Explanation
The statement "Gravitational force = Gm1m2/r^2" is true because it represents the formula for calculating the gravitational force between two objects. Here is a detailed explanation of each component of the formula:
1. G: The gravitational constant is denoted by G and has a value of approximately 6.67430 × 10^-11 N(m/kg)^2. It is a fundamental constant in physics that determines the strength of the gravitational force.
2. m1 and m2: These variables represent the masses of the two objects involved in the gravitational interaction. The gravitational force is directly proportional to the product of their masses. As the masses increase, the gravitational force between the objects also increases.
3. r: The distance between the centers of mass of the two objects is denoted by r. The gravitational force is inversely proportional to the square of the distance between the objects. As the distance increases, the gravitational force decreases.
4. F: The gravitational force is the force of attraction between the two objects. It acts along the line connecting their centers of mass and is always an attractive force.
Conclusion
In summary, the equation "Gravitational force = Gm1m2/r^2" is true and represents the formula for calculating the gravitational force between two objects. It takes into account the gravitational constant, the masses of the objects, and the distance between them. By understanding this formula, we can quantitatively analyze and predict the gravitational interactions between various objects.
What causes spectral lines?- a)The transition of electrons between two energy levels
- b)The transition of electrons between two wavelength ranges
- c)Magnetic and electric field exiting in an atom
- d)The transition of electrons from electric to magnetic field
Correct answer is option 'A'. Can you explain this answer?
What causes spectral lines?
a)
The transition of electrons between two energy levels
b)
The transition of electrons between two wavelength ranges
c)
Magnetic and electric field exiting in an atom
d)
The transition of electrons from electric to magnetic field
|
|
Vivek Khatri answered |
The observed spectral lines are caused by the transition of electrons between two energy levels in an atom. The emission spectrum of the hydrogen atom is divided into many spectral series, with wavelengths that are given by Rydberg’s formula.
dΨ/dx must be zero.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
dΨ/dx must be zero.
a)
True
b)
False
|
|
Vivek Khatri answered |
For a wave function, dΨ/dx, must be continuous and single-valued everywhere, just like Ψ. Also, Ψ must be normalizable.
Angular momentum of an electron is quantized.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
Angular momentum of an electron is quantized.
a)
True
b)
False
|
|
Vivek Khatri answered |
According to Bohr’s postulate, angular momentum is quantized and this is given by the expression mevr = nh/2π. (n =1, 2, 3….). mevr is the angular momentum and h is the Planck’s constant. Movement of an electron can only be possible in orbits whose angular momentum is the integral multiple of h/2π
The principal quantum number describes ____- a)energy and size of the orbit
- b)the shape of the orbital
- c)spatial orientation of the orbital
- d)the spin of the electron
Correct answer is option 'A'. Can you explain this answer?
The principal quantum number describes ____
a)
energy and size of the orbit
b)
the shape of the orbital
c)
spatial orientation of the orbital
d)
the spin of the electron

|
Raksha Pillai answered |
The principal quantum number describes energy and size of the orbit.
Explanation:
Principal Quantum Number (n) refers to the main energy level of an electron in an atom. It determines the energy and the size of the orbital where an electron is likely to be found. Here is a detailed explanation:
- Energy Level: The principal quantum number (n) indicates the energy level of an electron in an atom. The higher the value of n, the higher the energy level of the electron.
- Size of the Orbit: The principal quantum number also determines the size of the orbital or the distance of the electron from the nucleus. As the value of n increases, the distance of the electron from the nucleus also increases, leading to larger orbitals.
- Relationship to Atomic Structure: The principal quantum number plays a crucial role in understanding the electronic structure of an atom. It helps in determining the distribution of electrons in different energy levels and orbitals.
- Comparison with Other Quantum Numbers: While the principal quantum number describes the energy and size of the orbit, other quantum numbers such as the azimuthal quantum number (l) describe the shape of the orbital, the magnetic quantum number (m) describes the spatial orientation of the orbital, and the spin quantum number describes the spin of the electron.
In conclusion, the principal quantum number is a fundamental concept in quantum mechanics that helps in defining the energy levels and orbital sizes of electrons in an atom.
Explanation:
Principal Quantum Number (n) refers to the main energy level of an electron in an atom. It determines the energy and the size of the orbital where an electron is likely to be found. Here is a detailed explanation:
- Energy Level: The principal quantum number (n) indicates the energy level of an electron in an atom. The higher the value of n, the higher the energy level of the electron.
- Size of the Orbit: The principal quantum number also determines the size of the orbital or the distance of the electron from the nucleus. As the value of n increases, the distance of the electron from the nucleus also increases, leading to larger orbitals.
- Relationship to Atomic Structure: The principal quantum number plays a crucial role in understanding the electronic structure of an atom. It helps in determining the distribution of electrons in different energy levels and orbitals.
- Comparison with Other Quantum Numbers: While the principal quantum number describes the energy and size of the orbit, other quantum numbers such as the azimuthal quantum number (l) describe the shape of the orbital, the magnetic quantum number (m) describes the spatial orientation of the orbital, and the spin quantum number describes the spin of the electron.
In conclusion, the principal quantum number is a fundamental concept in quantum mechanics that helps in defining the energy levels and orbital sizes of electrons in an atom.
How many spectral lines are there in the hydrogen spectrum?- a)Infinity
- b)Zero
- c)Multiple
- d)One
Correct answer is option 'C'. Can you explain this answer?
How many spectral lines are there in the hydrogen spectrum?
a)
Infinity
b)
Zero
c)
Multiple
d)
One

|
Sarthak Chavan answered |
Spectral Lines in Hydrogen Spectrum
The hydrogen spectrum refers to the series of spectral lines that are produced when electrons in a hydrogen atom move from higher to lower energy levels. These transitions result in the emission of electromagnetic radiation, which can be detected and analyzed using spectroscopy.
Number of Spectral Lines
The number of spectral lines in the hydrogen spectrum depends on the number of energy levels that the electrons can occupy. In the case of hydrogen, there are an infinite number of energy levels, but only a finite number of these levels are accessible to the electrons.
The spectral lines that are produced in the hydrogen spectrum correspond to transitions between these accessible energy levels. Each transition corresponds to a specific wavelength of electromagnetic radiation, which can be detected as a spectral line.
Therefore, the correct answer to the question is option C, multiple. There are multiple spectral lines in the hydrogen spectrum, corresponding to the various transitions between energy levels.
Conclusion
In summary, the hydrogen spectrum is characterized by multiple spectral lines that correspond to transitions between energy levels in the atom. The number of spectral lines is finite but large, and each line corresponds to a specific wavelength of electromagnetic radiation. Understanding the hydrogen spectrum is important for a variety of scientific applications, including astronomy, chemistry, and physics.
The hydrogen spectrum refers to the series of spectral lines that are produced when electrons in a hydrogen atom move from higher to lower energy levels. These transitions result in the emission of electromagnetic radiation, which can be detected and analyzed using spectroscopy.
Number of Spectral Lines
The number of spectral lines in the hydrogen spectrum depends on the number of energy levels that the electrons can occupy. In the case of hydrogen, there are an infinite number of energy levels, but only a finite number of these levels are accessible to the electrons.
The spectral lines that are produced in the hydrogen spectrum correspond to transitions between these accessible energy levels. Each transition corresponds to a specific wavelength of electromagnetic radiation, which can be detected as a spectral line.
Therefore, the correct answer to the question is option C, multiple. There are multiple spectral lines in the hydrogen spectrum, corresponding to the various transitions between energy levels.
Conclusion
In summary, the hydrogen spectrum is characterized by multiple spectral lines that correspond to transitions between energy levels in the atom. The number of spectral lines is finite but large, and each line corresponds to a specific wavelength of electromagnetic radiation. Understanding the hydrogen spectrum is important for a variety of scientific applications, including astronomy, chemistry, and physics.
What’s the radius of 1st orbit of He+ atom?- a)0.1058 nm
- b)0.2156 nm
- c)0.00529 nm
- d)0.02645 nm
Correct answer is option 'D'. Can you explain this answer?
What’s the radius of 1st orbit of He+ atom?
a)
0.1058 nm
b)
0.2156 nm
c)
0.00529 nm
d)
0.02645 nm
|
|
Vivek Khatri answered |
The atomic radius of an atom is given by the formula rn = 52.9n2/Z pm, where rn is the radius of nth orbit of an atom and Z is the atomic number of that atom. For He+, n = 1 and Z =2. Radius = 52.9(1)/2 pm = 0.02645 nm.
Bohr’s model couldn’t explain Zeeman and stark effect.- a)False
- b)True
Correct answer is option 'B'. Can you explain this answer?
Bohr’s model couldn’t explain Zeeman and stark effect.
a)
False
b)
True
|
|
Vivek Khatri answered |
Yes, it’s a limitation of Bohr’s model that it could not the splitting of spectral lines in the magnetic field that is Zeeman effect and also in the electric field also known as a stark effect. so the above statement is true.
Which of the following models are not the same as Thomson Model of Atom?- a)plum pudding model
- b)watermelon model
- c)raisin pudding model
- d)nuclear model
Correct answer is option 'D'. Can you explain this answer?
Which of the following models are not the same as Thomson Model of Atom?
a)
plum pudding model
b)
watermelon model
c)
raisin pudding model
d)
nuclear model
|
|
Vivek Khatri answered |
Thomson proposed a model of the atom, in which electrons are embedded to make it as the stable electrostatic arrangement and such that positive charge is equally distributed around a sphere. Mass is assumed to be equally distributed. So. it has different names like plum pudding, watermelon and raisin pudding model.
What is the ratio of the atomic radius of the 5th orbit in chlorine atom and 3rd orbit in Helium atom?- a)153 : 50
- b)50 : 153
- c)153 : 100
- d)100 : 153
Correct answer is option 'B'. Can you explain this answer?
What is the ratio of the atomic radius of the 5th orbit in chlorine atom and 3rd orbit in Helium atom?
a)
153 : 50
b)
50 : 153
c)
153 : 100
d)
100 : 153
|
|
Vivek Khatri answered |
The atomic radius of an atom is given by the formula rn = 52.9n2/Z pm, where rn is the radius of nth orbit of an atom and Z is the atomic number of that atom. The ratio of the atomic radius of the 5th orbit in chlorine atom and 3rd orbit in Helium atom is 25/17 : 9/2 = 50 : 153.
Bohr’s model only works for hydrogen or helium.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
Bohr’s model only works for hydrogen or helium.
a)
True
b)
False

|
Palak Singh answered |
Niels Bohr was a Danish physicist who made significant contributions to the field of quantum mechanics and atomic theory. He is best known for his model of the atom, known as the Bohr model, which proposed that electrons orbit the nucleus in fixed energy levels or shells. This model helped to explain the behavior of electrons and their emission and absorption of energy.
Bohr also made important contributions to the understanding of nuclear fission and the development of the atomic bomb. He worked on the Manhattan Project during World War II, where he collaborated with other scientists to develop the first atomic weapons. However, he later became an advocate for peaceful use of atomic energy and worked to promote international cooperation in the field of nuclear science.
In recognition of his contributions, Niels Bohr was awarded the Nobel Prize in Physics in 1922 for his research on the structure of atoms and the radiation they emit. He continued to be active in scientific research and international diplomacy until his death in 1962.
Bohr also made important contributions to the understanding of nuclear fission and the development of the atomic bomb. He worked on the Manhattan Project during World War II, where he collaborated with other scientists to develop the first atomic weapons. However, he later became an advocate for peaceful use of atomic energy and worked to promote international cooperation in the field of nuclear science.
In recognition of his contributions, Niels Bohr was awarded the Nobel Prize in Physics in 1922 for his research on the structure of atoms and the radiation they emit. He continued to be active in scientific research and international diplomacy until his death in 1962.
No two electrons have the same set of all four quantum numbers.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
No two electrons have the same set of all four quantum numbers.
a)
True
b)
False
|
|
Vivek Khatri answered |
Yes, no two electrons have the same set of all four quantum numbers. This is explained by Pauli’s exclusive principle. At most electrons can have all 3 quantum numbers the same as they are in the same orbital. But the spin quantum number’s values are different.
Which of the following is the correct expression for the Schrödinger wave function?- a)
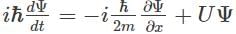
- b)
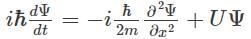
- c)
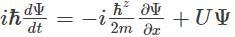
- d)
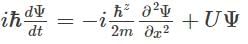
Correct answer is option 'D'. Can you explain this answer?
Which of the following is the correct expression for the Schrödinger wave function?
a)
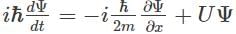
b)
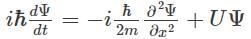
c)
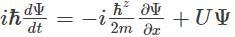
d)
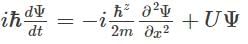

|
Edurev.iitjam answered |
The correct expression for the Schrödinger wave equation is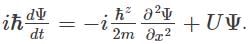 Schrodinger equation is a basic principle in itself.
Schrodinger equation is a basic principle in itself.
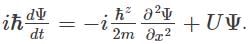 Schrodinger equation is a basic principle in itself.
Schrodinger equation is a basic principle in itself.Chemical properties of an atom are dependent on a number of electrons in that particular atom.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
Chemical properties of an atom are dependent on a number of electrons in that particular atom.
a)
True
b)
False

|
Jay Nambiar answered |
Explanation:
Number of Electrons and Chemical Properties:
- The number of electrons in an atom determines its chemical properties.
- Electrons play a crucial role in the bonding behavior of atoms, which in turn determines how atoms interact with each other to form compounds.
- The arrangement of electrons in an atom's electron shells influences its reactivity and ability to form bonds with other atoms.
- For example, atoms with a full outer electron shell (such as noble gases) tend to be stable and unreactive, while atoms with incomplete outer shells are more likely to form bonds in order to achieve a stable electron configuration.
Effect on Reactivity:
- The number of electrons in an atom's outer shell determines its reactivity.
- Atoms with fewer electrons in their outer shell tend to lose, gain, or share electrons to achieve a stable electron configuration, resulting in the formation of chemical bonds.
- The reactivity of an atom is also influenced by factors such as electronegativity, electron affinity, and ionization energy, which are all related to the number and arrangement of electrons in the atom.
Conclusion:
In conclusion, the chemical properties of an atom are indeed dependent on the number of electrons in that atom. The arrangement of electrons in an atom's electron shells influences its reactivity, bonding behavior, and overall chemical properties. Understanding the role of electrons in atoms is essential in predicting how different elements will interact with each other to form compounds.
Number of Electrons and Chemical Properties:
- The number of electrons in an atom determines its chemical properties.
- Electrons play a crucial role in the bonding behavior of atoms, which in turn determines how atoms interact with each other to form compounds.
- The arrangement of electrons in an atom's electron shells influences its reactivity and ability to form bonds with other atoms.
- For example, atoms with a full outer electron shell (such as noble gases) tend to be stable and unreactive, while atoms with incomplete outer shells are more likely to form bonds in order to achieve a stable electron configuration.
Effect on Reactivity:
- The number of electrons in an atom's outer shell determines its reactivity.
- Atoms with fewer electrons in their outer shell tend to lose, gain, or share electrons to achieve a stable electron configuration, resulting in the formation of chemical bonds.
- The reactivity of an atom is also influenced by factors such as electronegativity, electron affinity, and ionization energy, which are all related to the number and arrangement of electrons in the atom.
Conclusion:
In conclusion, the chemical properties of an atom are indeed dependent on the number of electrons in that atom. The arrangement of electrons in an atom's electron shells influences its reactivity, bonding behavior, and overall chemical properties. Understanding the role of electrons in atoms is essential in predicting how different elements will interact with each other to form compounds.
Any wave function can be written as a linear combination of ______- a)Eigen Vectors
- b)Eigen Values
- c)Eigen Functions
- d)Operators
Correct answer is option 'C'. Can you explain this answer?
Any wave function can be written as a linear combination of ______
a)
Eigen Vectors
b)
Eigen Values
c)
Eigen Functions
d)
Operators
|
|
Vivek Khatri answered |
A wave function describes the state of a particle. It does not have a physical significance. Moreover, it can be written as a linear combination of Eigen functions, i.e., Ψ(x) = AF(x) + BG(x).
Which of the following is not a characteristic of wave function?- a)Continuous
- b)Single valued
- c)Differentiable
- d)Physically Significant
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not a characteristic of wave function?
a)
Continuous
b)
Single valued
c)
Differentiable
d)
Physically Significant

|
Tarun Singh answered |
Wave function and its characteristics
The wave function is a fundamental concept in quantum mechanics that describes the behavior of particles. It is a mathematical function that represents the probability amplitude of finding a particle in a particular state. The wave function is typically denoted by the Greek letter psi (ψ).
Characteristics of wave function:
a) Continuous: The wave function is continuous, meaning that it is defined for all values of the independent variables. In other words, it does not have any discontinuities or jumps in its values.
b) Single valued: The wave function is single valued, which means that it gives a unique value for each point in space and time. This ensures that the probability of finding a particle at a specific location is well-defined.
c) Differentiable: The wave function is differentiable, meaning that it can be differentiated with respect to its independent variables (position, time, etc.). This allows us to calculate various physical quantities, such as momentum and energy, associated with the particle.
d) Physically Significant: This characteristic is not associated with the wave function itself. Rather, it refers to the physical interpretation and significance of the wave function. The wave function provides information about the probability distribution of a particle's position, momentum, and other observables. It is a mathematical tool used to describe quantum systems and their behavior.
Explanation of the correct answer:
The correct answer is option 'D' - Physically Significant. This is because the wave function, by itself, does not have any physical significance. It is a mathematical construct that allows us to calculate probabilities and other observables associated with quantum systems. The physical significance arises when the wave function is used to calculate the probability density of finding a particle at a specific location or the expectation value of an observable.
In quantum mechanics, the physical significance is attributed to the square of the wave function, known as the probability density. The probability density represents the likelihood of finding a particle at a specific position. It is obtained by taking the absolute square of the wave function.
Therefore, while the wave function itself is a key mathematical tool in quantum mechanics, its physical significance lies in its relationship to the probability density and the ability to calculate various observable quantities.
The wave function is a fundamental concept in quantum mechanics that describes the behavior of particles. It is a mathematical function that represents the probability amplitude of finding a particle in a particular state. The wave function is typically denoted by the Greek letter psi (ψ).
Characteristics of wave function:
a) Continuous: The wave function is continuous, meaning that it is defined for all values of the independent variables. In other words, it does not have any discontinuities or jumps in its values.
b) Single valued: The wave function is single valued, which means that it gives a unique value for each point in space and time. This ensures that the probability of finding a particle at a specific location is well-defined.
c) Differentiable: The wave function is differentiable, meaning that it can be differentiated with respect to its independent variables (position, time, etc.). This allows us to calculate various physical quantities, such as momentum and energy, associated with the particle.
d) Physically Significant: This characteristic is not associated with the wave function itself. Rather, it refers to the physical interpretation and significance of the wave function. The wave function provides information about the probability distribution of a particle's position, momentum, and other observables. It is a mathematical tool used to describe quantum systems and their behavior.
Explanation of the correct answer:
The correct answer is option 'D' - Physically Significant. This is because the wave function, by itself, does not have any physical significance. It is a mathematical construct that allows us to calculate probabilities and other observables associated with quantum systems. The physical significance arises when the wave function is used to calculate the probability density of finding a particle at a specific location or the expectation value of an observable.
In quantum mechanics, the physical significance is attributed to the square of the wave function, known as the probability density. The probability density represents the likelihood of finding a particle at a specific position. It is obtained by taking the absolute square of the wave function.
Therefore, while the wave function itself is a key mathematical tool in quantum mechanics, its physical significance lies in its relationship to the probability density and the ability to calculate various observable quantities.
Write the values for l, n, and m for Ψ3,1,0?
- a)1, 3, 0
- b)3, 1, 0
- c)0, 3, 1
- d)1, 0, 3
Correct answer is option 'A'. Can you explain this answer?
Write the values for l, n, and m for Ψ3,1,0?
a)
1, 3, 0
b)
3, 1, 0
c)
0, 3, 1
d)
1, 0, 3

|
Pranavi Mishra answered |
Sorry, the question is incomplete. Please provide more information or context so I can assist you better.
How many electrons can exist with the principal quantum number’s value as 4?- a)16
- b)4
- c)32
- d)12
Correct answer is option 'C'. Can you explain this answer?
How many electrons can exist with the principal quantum number’s value as 4?
a)
16
b)
4
c)
32
d)
12
|
|
Vivek Khatri answered |
The number of orbitals within an orbit is n2. But as each orbital can accommodate 2 electrons, the number of electrons that can exist with the “n” as the principal quantum number is 2n2. So here 2n2 = 2(4)2 = 2(16) = 32.
Find out the number of neutrons, protons, and electrons of 17Cl37 respectively.- a)20, 20, 17
- b)17, 17, 20
- c)20, 17, 17
- d)17, 17, 17
Correct answer is option 'C'. Can you explain this answer?
Find out the number of neutrons, protons, and electrons of 17Cl37 respectively.
a)
20, 20, 17
b)
17, 17, 20
c)
20, 17, 17
d)
17, 17, 17

|
Shubham Rane answered |
Number of neutrons, protons, and electrons in 17Cl37:
To determine the number of neutrons, protons, and electrons in an atom, we need to understand the atomic symbol notation and the concept of atomic number and mass number.
- Atomic symbol notation:
The atomic symbol notation consists of the element symbol, atomic number, and mass number. For example, in the case of 17Cl37, "Cl" represents the element chlorine, "17" represents the atomic number, and "37" represents the mass number.
- Atomic number:
The atomic number (Z) represents the number of protons in the nucleus of an atom. It also determines the identity of the element. In this case, the atomic number of chlorine is 17.
- Mass number:
The mass number (A) represents the total number of protons and neutrons present in the nucleus of an atom. In this case, the mass number of chlorine is 37.
To find the number of neutrons, protons, and electrons in 17Cl37, we can use the following formulas:
- Number of neutrons = Mass number - Atomic number
- Number of protons = Atomic number
- Number of electrons = Number of protons
Calculations:
Using the formulas mentioned above, let's calculate the number of neutrons, protons, and electrons in 17Cl37.
- Number of neutrons = 37 - 17 = 20
- Number of protons = 17
- Number of electrons = 17
Therefore, the number of neutrons, protons, and electrons in 17Cl37 is 20, 17, and 17, respectively.
Summary:
The correct answer is option 'C' (20, 17, 17). Chlorine-37 (17Cl37) has 20 neutrons, 17 protons, and 17 electrons.
To determine the number of neutrons, protons, and electrons in an atom, we need to understand the atomic symbol notation and the concept of atomic number and mass number.
- Atomic symbol notation:
The atomic symbol notation consists of the element symbol, atomic number, and mass number. For example, in the case of 17Cl37, "Cl" represents the element chlorine, "17" represents the atomic number, and "37" represents the mass number.
- Atomic number:
The atomic number (Z) represents the number of protons in the nucleus of an atom. It also determines the identity of the element. In this case, the atomic number of chlorine is 17.
- Mass number:
The mass number (A) represents the total number of protons and neutrons present in the nucleus of an atom. In this case, the mass number of chlorine is 37.
To find the number of neutrons, protons, and electrons in 17Cl37, we can use the following formulas:
- Number of neutrons = Mass number - Atomic number
- Number of protons = Atomic number
- Number of electrons = Number of protons
Calculations:
Using the formulas mentioned above, let's calculate the number of neutrons, protons, and electrons in 17Cl37.
- Number of neutrons = 37 - 17 = 20
- Number of protons = 17
- Number of electrons = 17
Therefore, the number of neutrons, protons, and electrons in 17Cl37 is 20, 17, and 17, respectively.
Summary:
The correct answer is option 'C' (20, 17, 17). Chlorine-37 (17Cl37) has 20 neutrons, 17 protons, and 17 electrons.
Schrodinger Wave equation can be derived from Principles of Quantum Mechanics.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
Schrodinger Wave equation can be derived from Principles of Quantum Mechanics.
a)
True
b)
False

|
Rishabh Mehta answered |
False
The Schrödinger Wave Equation cannot be derived from the principles of quantum mechanics. The equation was actually formulated by Erwin Schrödinger in 1925, a few years after the development of the principles of quantum mechanics by pioneers such as Max Planck and Albert Einstein.
Principles of Quantum Mechanics
- Quantum mechanics is a branch of physics that describes the behavior of particles at the atomic and subatomic levels.
- It is based on several fundamental principles, including the wave-particle duality, the uncertainty principle, and the superposition principle.
- These principles were developed through experimental observations and theoretical calculations by various scientists.
Schrödinger Wave Equation
- The Schrödinger Wave Equation is a mathematical equation that describes the behavior of quantum-mechanical systems, such as electrons in atoms.
- It is a partial differential equation that relates the wave function of a system to its energy and potential energy.
- The wave function, represented by the Greek letter psi (ψ), describes the probability amplitude of finding a particle in a particular state.
- The equation is named after its creator, Erwin Schrödinger, who developed it as an alternative formulation of quantum mechanics.
Derivation of the Schrödinger Wave Equation
- The Schrödinger Wave Equation is derived through a mathematical process known as the variational principle.
- The variational principle involves minimizing the expectation value of the energy of a system with respect to its wave function.
- This leads to the derivation of the time-independent Schrödinger Wave Equation, which describes stationary states with definite energy values.
- The time-dependent Schrödinger Wave Equation, which describes the time evolution of quantum systems, can be derived from the time-independent equation.
Conclusion
In conclusion, the Schrödinger Wave Equation cannot be derived from the principles of quantum mechanics. It was developed as a mathematical formulation by Erwin Schrödinger to describe the behavior of quantum-mechanical systems. The equation is a fundamental tool in quantum mechanics and is used to calculate the wave function and energy of particles in various physical systems.
The Schrödinger Wave Equation cannot be derived from the principles of quantum mechanics. The equation was actually formulated by Erwin Schrödinger in 1925, a few years after the development of the principles of quantum mechanics by pioneers such as Max Planck and Albert Einstein.
Principles of Quantum Mechanics
- Quantum mechanics is a branch of physics that describes the behavior of particles at the atomic and subatomic levels.
- It is based on several fundamental principles, including the wave-particle duality, the uncertainty principle, and the superposition principle.
- These principles were developed through experimental observations and theoretical calculations by various scientists.
Schrödinger Wave Equation
- The Schrödinger Wave Equation is a mathematical equation that describes the behavior of quantum-mechanical systems, such as electrons in atoms.
- It is a partial differential equation that relates the wave function of a system to its energy and potential energy.
- The wave function, represented by the Greek letter psi (ψ), describes the probability amplitude of finding a particle in a particular state.
- The equation is named after its creator, Erwin Schrödinger, who developed it as an alternative formulation of quantum mechanics.
Derivation of the Schrödinger Wave Equation
- The Schrödinger Wave Equation is derived through a mathematical process known as the variational principle.
- The variational principle involves minimizing the expectation value of the energy of a system with respect to its wave function.
- This leads to the derivation of the time-independent Schrödinger Wave Equation, which describes stationary states with definite energy values.
- The time-dependent Schrödinger Wave Equation, which describes the time evolution of quantum systems, can be derived from the time-independent equation.
Conclusion
In conclusion, the Schrödinger Wave Equation cannot be derived from the principles of quantum mechanics. It was developed as a mathematical formulation by Erwin Schrödinger to describe the behavior of quantum-mechanical systems. The equation is a fundamental tool in quantum mechanics and is used to calculate the wave function and energy of particles in various physical systems.
The energy of 1st orbit in a hydrogen atom __________- a)3.18×10–12 J
- b)–2.18×10–18 J
- c)–3.18×10–18 J
- d)2.18×10–18 J
Correct answer is option 'B'. Can you explain this answer?
The energy of 1st orbit in a hydrogen atom __________
a)
3.18×10–12 J
b)
–2.18×10–18 J
c)
–3.18×10–18 J
d)
2.18×10–18 J

|
Sneha Menon answered |
The energy of 1st orbit in a hydrogen atom is -13.6 eV.
The Schrödinger is a differential equation.- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
The Schrödinger is a differential equation.
a)
True
b)
False

|
Yashvi Roy answered |
The Schrödinger equation is a partial differential equation that describes how the wave function of a physical system changes over time. It was developed by Austrian physicist Erwin Schrödinger in 1925 as part of the development of quantum mechanics.
The equation is written as:
iħ∂ψ/∂t = -ħ²/2m∇²ψ + Vψ
where i is the imaginary unit, ħ is the reduced Planck constant, t is time, ψ is the wave function, m is the mass of the particle, ∇² is the Laplacian operator, V is the potential energy, and ∂/∂t is the partial derivative with respect to time.
The Schrödinger equation describes the behavior of quantum systems, which are characterized by wave-particle duality. The wave function ψ represents the probability amplitude of finding a particle in a particular state. The term -ħ²/2m∇²ψ represents the kinetic energy of the particle, while Vψ represents the potential energy.
Solving the Schrödinger equation allows us to determine the wave function and hence calculate properties of the system, such as energy levels and probabilities of different outcomes. It provides a mathematical framework for understanding the behavior of quantum particles, and has been successful in explaining a wide range of phenomena, from the behavior of electrons in atoms to the formation of molecular bonds.
The equation is written as:
iħ∂ψ/∂t = -ħ²/2m∇²ψ + Vψ
where i is the imaginary unit, ħ is the reduced Planck constant, t is time, ψ is the wave function, m is the mass of the particle, ∇² is the Laplacian operator, V is the potential energy, and ∂/∂t is the partial derivative with respect to time.
The Schrödinger equation describes the behavior of quantum systems, which are characterized by wave-particle duality. The wave function ψ represents the probability amplitude of finding a particle in a particular state. The term -ħ²/2m∇²ψ represents the kinetic energy of the particle, while Vψ represents the potential energy.
Solving the Schrödinger equation allows us to determine the wave function and hence calculate properties of the system, such as energy levels and probabilities of different outcomes. It provides a mathematical framework for understanding the behavior of quantum particles, and has been successful in explaining a wide range of phenomena, from the behavior of electrons in atoms to the formation of molecular bonds.
If the number of protons and neutrons of an element is 13 and 14 respectively, then what’s the atomic number(Z) and mass number(A)?- a)13, 13
- b)13, 27
- c)14, 13
- d)27, 14
Correct answer is option 'B'. Can you explain this answer?
If the number of protons and neutrons of an element is 13 and 14 respectively, then what’s the atomic number(Z) and mass number(A)?
a)
13, 13
b)
13, 27
c)
14, 13
d)
27, 14

|
Bhavana Dasgupta answered |
The number of protons and neutrons of an element is 13 and 14 respectively.
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Which of the following properties of atom could be explained correctly by Thomson Model of atom?- a)Overall neutrality of atom.
- b)Spectra of hydrogen atom.
- c)Position of electrons, protons and neutrons in atom.
- d)Stability of atom.
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Which of the following properties of atom could be explained correctly by Thomson Model of atom?
a)
Overall neutrality of atom.
b)
Spectra of hydrogen atom.
c)
Position of electrons, protons and neutrons in atom.
d)
Stability of atom.
|
|
Anjana Sharma answered |
According to Thomson model of atom, the mass of the atom is assumed to be uniformly distributed over the atom. This model was able to explain the overall neutrality of the atom.
Chapter doubts & questions for Atomic and Molecular Structure - Physical Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Atomic and Molecular Structure - Physical Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Physical Chemistry
83 videos|142 docs|67 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily












