All Exams >
JEE >
JEE Main & Advanced Mock Test Series 2025 >
All Questions
All questions of JEE Main & Advanced (2015) for JEE Exam
The synthesis of alkyl fluorides is best accomplished by- a)Free radical fluorination
- b)Sandmeyer's reaction
- c)Finkelstein reaction
- d)Swarts reaction
Correct answer is option 'D'. Can you explain this answer?
The synthesis of alkyl fluorides is best accomplished by
a)
Free radical fluorination
b)
Sandmeyer's reaction
c)
Finkelstein reaction
d)
Swarts reaction
|
|
T.ttttt answered |
The synthesis of alkyl fluorides is best accomplished by Swarts reaction.
Alkyl bromides or alkyl chlorides are heated in presence of metallifluorides such as AgF,CoF2,SbF5orHg2F2 to obtain alkyl fluorides.
CH3−Cl+AgF→CH3−F+AgCl
Alkyl bromides or alkyl chlorides are heated in presence of metallifluorides such as AgF,CoF2,SbF5orHg2F2 to obtain alkyl fluorides.
CH3−Cl+AgF→CH3−F+AgCl
A block of mass m =10 kg rests on a horizontal table. The coefficient of friction between the block and the table is 0.05. When hit by a bullet of mass 50 g moving with speed v, that gets embedded in it, the block moves and comes to stop after moving a distance of 2 m on the table. If a freely falling object were to acquire speed v/10 after being dropped from height H, then neglecting energy losses and taking g = 10 ms-2 , the value of H is close to :- a)0.2 km
- b)0.3 km
- c)0.4 km
- d)0.5 km
Correct answer is option 'C'. Can you explain this answer?
A block of mass m =10 kg rests on a horizontal table. The coefficient of friction between the block and the table is 0.05. When hit by a bullet of mass 50 g moving with speed v, that gets embedded in it, the block moves and comes to stop after moving a distance of 2 m on the table. If a freely falling object were to acquire speed v/10 after being dropped from height H, then neglecting energy losses and taking g = 10 ms-2 , the value of H is close to :
a)
0.2 km
b)
0.3 km
c)
0.4 km
d)
0.5 km
|
|
Krishna Iyer answered |
Momentum conservation 0.05V=10V0

None of the options match.

None of the options match.
For a simple pendulum, a graph is plotted between its kinetic energy (KE) and potential energy (PE) against its displacement d. Which one of the following represents these correctly?
(Graphs are schematic and not drawn to scale)- a)
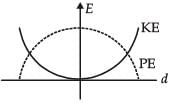
- b)
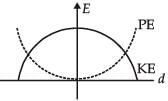
- c)
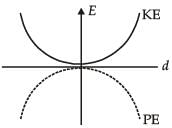
- d)
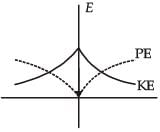
Correct answer is option 'B'. Can you explain this answer?
For a simple pendulum, a graph is plotted between its kinetic energy (KE) and potential energy (PE) against its displacement d. Which one of the following represents these correctly?
(Graphs are schematic and not drawn to scale)
(Graphs are schematic and not drawn to scale)
a)

b)

c)

d)

|
|
Krishna Iyer answered |
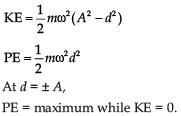
The energy of a system as a function of time t is given as E(t) = A2exp(-αt), where α = 0.2 s-1. The
measurement of A has an error of 1.25 %. If the error in the measurement of time is 1.50 %, the percentage
error in the value of E(t) at t = 5 s is
Correct answer is '4'. Can you explain this answer?
The energy of a system as a function of time t is given as E(t) = A2exp(-αt), where α = 0.2 s-1. The
measurement of A has an error of 1.25 %. If the error in the measurement of time is 1.50 %, the percentage
error in the value of E(t) at t = 5 s is
measurement of A has an error of 1.25 %. If the error in the measurement of time is 1.50 %, the percentage
error in the value of E(t) at t = 5 s is

|
Sushil Kumar answered |
A signal of 5 kHz frequency is amplitude modulated on a carrier wave of frequency 2 MHz. The frequencies of the resultant signal is/are- a)2 MHz only
- b)2005 kHz and 1995 kHz
- c)2005 kHz, 2000 kHz and 1995 kHz
- d)2000 kHz and 1995 kHz
Correct answer is option 'C'. Can you explain this answer?
A signal of 5 kHz frequency is amplitude modulated on a carrier wave of frequency 2 MHz. The frequencies of the resultant signal is/are
a)
2 MHz only
b)
2005 kHz and 1995 kHz
c)
2005 kHz, 2000 kHz and 1995 kHz
d)
2000 kHz and 1995 kHz
|
|
Md Jisan answered |
C, if the frequency of the carrier wave is Fc and the frequency of the modulating wave is Fm then we know that the original will be the same as Fc and the side bands will be (Fc+Fm)and (Fc -Fm) ,so finally we will able to get the solution
The optically inactive compound from the following is :- a)2 - chloropropanal
- b)2 - chloropentane
- c)2 - chlorobutane
- d)2 - chloro - 2 - methylbutane
Correct answer is option 'D'. Can you explain this answer?
The optically inactive compound from the following is :
a)
2 - chloropropanal
b)
2 - chloropentane
c)
2 - chlorobutane
d)
2 - chloro - 2 - methylbutane

|
Nirmal answered |
C2 has attached two methyl group. so ans is (d)
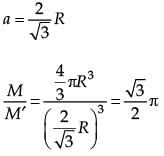
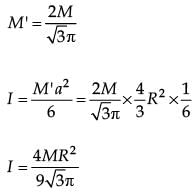
From a solid sphere of mass M and radius R, a spherical portion of radius R/2 is removed, as shown in the figure. Taking gravitational potential V = 0 at r = ∞, the potential at the centre of the cavity thus formed is (G = gravitational constant)

- a)

- b)
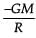
- c)
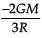
- d)
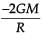
Correct answer is option 'B'. Can you explain this answer?
From a solid sphere of mass M and radius R, a spherical portion of radius R/2 is removed, as shown in the figure. Taking gravitational potential V = 0 at r = ∞, the potential at the centre of the cavity thus formed is (G = gravitational constant)


a)

b)
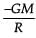
c)
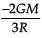
d)
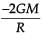
|
|
Lavanya Menon answered |
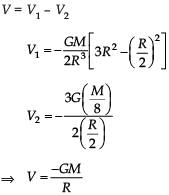
Match List-I (Fundamental Experiment) with List-II (its conclusion) and select the correct option from the choices given below the list:
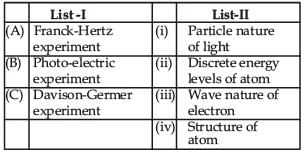
- a)(A) - (i) (B) - (iv) (C) - (iii)
- b)(A) - (ii) (B) - (iv) (C) - (iii)
- c)(A) - (ii) (B) - (i) (C) - (iii)
- d)(A) - (iv) (B) - (iii) (C) - (ii)
Correct answer is option 'C'. Can you explain this answer?
Match List-I (Fundamental Experiment) with List-II (its conclusion) and select the correct option from the choices given below the list:


a)
(A) - (i) (B) - (iv) (C) - (iii)
b)
(A) - (ii) (B) - (iv) (C) - (iii)
c)
(A) - (ii) (B) - (i) (C) - (iii)
d)
(A) - (iv) (B) - (iii) (C) - (ii)
|
|
Geetika Shah answered |
1. Franck-Hertz exp.– Discrete energy level.
2. Photo-electric effect– Particle nature of light
3. Davison-Germer exp.– Diffraction of electron beam.
2. Photo-electric effect– Particle nature of light
3. Davison-Germer exp.– Diffraction of electron beam.
Suppose that the foci of the ellipse 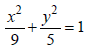 are (f1, 0) and (f2, 0) where f1 > 0 and f2 < 0. Let P1 and P2
are (f1, 0) and (f2, 0) where f1 > 0 and f2 < 0. Let P1 and P2
be two parabolas with a common vertex at (0, 0) and with foci at (f1, 0) and (2f2, 0), respectively. Let T1 be
a tangent to P1 which passes through (2f2, 0) and T2 be a tangent to P2 which passes through (f1, 0). The m1
is the slope of T1 and m2 is the slope of T2, then the value of 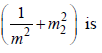
Correct answer is '4'. Can you explain this answer?
Suppose that the foci of the ellipse 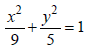 are (f1, 0) and (f2, 0) where f1 > 0 and f2 < 0. Let P1 and P2
are (f1, 0) and (f2, 0) where f1 > 0 and f2 < 0. Let P1 and P2
be two parabolas with a common vertex at (0, 0) and with foci at (f1, 0) and (2f2, 0), respectively. Let T1 be
a tangent to P1 which passes through (2f2, 0) and T2 be a tangent to P2 which passes through (f1, 0). The m1
is the slope of T1 and m2 is the slope of T2, then the value of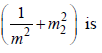
be two parabolas with a common vertex at (0, 0) and with foci at (f1, 0) and (2f2, 0), respectively. Let T1 be
a tangent to P1 which passes through (2f2, 0) and T2 be a tangent to P2 which passes through (f1, 0). The m1
is the slope of T1 and m2 is the slope of T2, then the value of
|
|
Anaya Patel answered |
A large spherical mass M is fixed at one position and two identical point masses m are kept on a line
passing through the centre of M (see figure). The point masses are connected by a rigid massless rod of
length 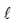 and this assembly is free to move along the line connecting them. All three masses interact only
and this assembly is free to move along the line connecting them. All three masses interact only
through their mutual gravitational interaction. When the point mass nearer to M is at a distance r = 3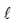 from
from
M, the tension in the rod is zero for m = k(M/288). The value of k is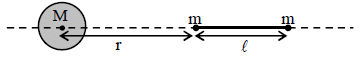
Correct answer is '7'. Can you explain this answer?
A large spherical mass M is fixed at one position and two identical point masses m are kept on a line
passing through the centre of M (see figure). The point masses are connected by a rigid massless rod of
length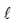 and this assembly is free to move along the line connecting them. All three masses interact only
and this assembly is free to move along the line connecting them. All three masses interact only
through their mutual gravitational interaction. When the point mass nearer to M is at a distance r = 3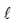 from
from
M, the tension in the rod is zero for m = k(M/288). The value of k is
passing through the centre of M (see figure). The point masses are connected by a rigid massless rod of
length
through their mutual gravitational interaction. When the point mass nearer to M is at a distance r = 3
M, the tension in the rod is zero for m = k(M/288). The value of k is
|
|
Krishna Iyer answered |
The vapour pressure of acetone at 20°C is 185 torr. When 1.2 g of a non-volatile substance was dissolved in 100 g of acetone at 20°C, its vapour pressure was 183 torr. The molar mass (g mol-1) of the substance is- a)32
- b)64
- c)128
- d) 488
Correct answer is option 'B'. Can you explain this answer?
The vapour pressure of acetone at 20°C is 185 torr. When 1.2 g of a non-volatile substance was dissolved in 100 g of acetone at 20°C, its vapour pressure was 183 torr. The molar mass (g mol-1) of the substance is
a)
32
b)
64
c)
128
d)
488
|
|
Anand Kumar answered |

parallel plate capacitor having plates of area S and plate separation d, has capacitance C1 in air. When
two dielectrics of different relative permittivities (ε1 = 2 and ε2 = 4) are introduced between the two plates
as shown in the figure, the capacitance becomes C2. The ratio C2/C1 is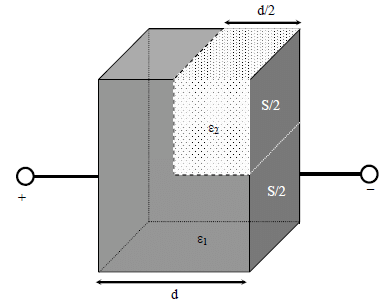
- a)6/5
- b)5/3
- c)7/5
- d)7/3
Correct answer is option 'D'. Can you explain this answer?
parallel plate capacitor having plates of area S and plate separation d, has capacitance C1 in air. When
two dielectrics of different relative permittivities (ε1 = 2 and ε2 = 4) are introduced between the two plates
as shown in the figure, the capacitance becomes C2. The ratio C2/C1 is
two dielectrics of different relative permittivities (ε1 = 2 and ε2 = 4) are introduced between the two plates
as shown in the figure, the capacitance becomes C2. The ratio C2/C1 is
a)
6/5
b)
5/3
c)
7/5
d)
7/3
|
|
Preeti Iyer answered |
The % yield of ammonia as a function of time in the reaction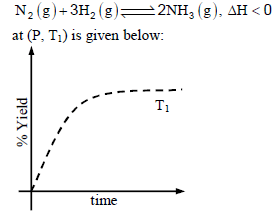 If this reaction is conducted at (P, T2), with T2 > T1, the % yield of ammonia as a function of time is
If this reaction is conducted at (P, T2), with T2 > T1, the % yield of ammonia as a function of time is
represented by- a)
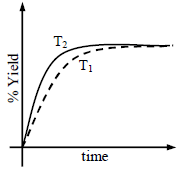
- b)
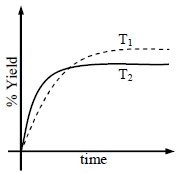
- c)
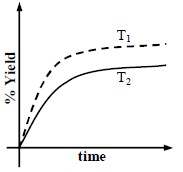
- d)
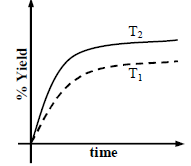
Correct answer is option 'B'. Can you explain this answer?
The % yield of ammonia as a function of time in the reaction
If this reaction is conducted at (P, T2), with T2 > T1, the % yield of ammonia as a function of time is
represented by
represented by
a)
b)
c)
d)
|
|
Krishna Iyer answered |
Increasing the temperature lowers equilibrium yield of ammonia.
However, at higher temperature the initial rate of forward reaction would be greater than at lower
temperature that is why the percentage yield of NH3 too would be more initially.
However, at higher temperature the initial rate of forward reaction would be greater than at lower
temperature that is why the percentage yield of NH3 too would be more initially.
Let the curve C be the mirror image of the parabola y2 = 4x with respect to the line x + y + 4 = 0. If A and B
are the points of intersection of C with the line y = -5, then the distance between A and B is
Correct answer is '4'. Can you explain this answer?
Let the curve C be the mirror image of the parabola y2 = 4x with respect to the line x + y + 4 = 0. If A and B
are the points of intersection of C with the line y = -5, then the distance between A and B is
are the points of intersection of C with the line y = -5, then the distance between A and B is
|
|
Chirag Verma answered |
Image of y = –5 about the line x + y + 4 = 0 is x = 1
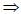 Distance AB = 4
Distance AB = 4
The number of geometric isomers that can exist for square planar [Pt(Cl)(py)(NH3)(NH2OH)]+ is (py = pyridine)- a)2
- b)3
- c)4
- d)6
Correct answer is option 'B'. Can you explain this answer?
The number of geometric isomers that can exist for square planar [Pt(Cl)(py)(NH3)(NH2OH)]+ is (py = pyridine)
a)
2
b)
3
c)
4
d)
6
|
|
Janhavi Sengupta answered |
as per question a = Cl, b = py, c = NH3 and d = NH2OH are assumed.
The set of all values of l for which the system of linear equations
2x1 - 2x2 + x3 = λx1
2x1 - 3x2 + 2x3 = λx2
-x1 + 2x2 = λx3
has a non-trivial solution- a)Is an empty set
- b)Is a singleton
- c)Contains two elements
- d)Contains more than two elements
Correct answer is option 'C'. Can you explain this answer?
The set of all values of l for which the system of linear equations
2x1 - 2x2 + x3 = λx1
2x1 - 3x2 + 2x3 = λx2
-x1 + 2x2 = λx3
has a non-trivial solution
2x1 - 2x2 + x3 = λx1
2x1 - 3x2 + 2x3 = λx2
-x1 + 2x2 = λx3
has a non-trivial solution
a)
Is an empty set
b)
Is a singleton
c)
Contains two elements
d)
Contains more than two elements

|
Peter Parker answered |
Option C is correct.
Which among the following is the most reactive?- a)Cl2
- b)Br2
- c)I2
- d)ICl
Correct answer is option 'D'. Can you explain this answer?
Which among the following is the most reactive?
a)
Cl2
b)
Br2
c)
I2
d)
ICl
|
|
Vivek Patel answered |
Because of polarity and weak bond interhalogen compounds are more reactive.
Which of the following compounds is not colored yellow?- a)Zn2[Fe(CN)6]
- b)K3[Co(NO2)6]
- c)(NH4)3[As (Mo3O10)4]
- d)BaCrO4
Correct answer is option 'A'. Can you explain this answer?
Which of the following compounds is not colored yellow?
a)
Zn2[Fe(CN)6]
b)
K3[Co(NO2)6]
c)
(NH4)3[As (Mo3O10)4]
d)
BaCrO4
|
|
Chirag Verma answered |
(NH4)3[As (Mo3O10)4], BaCrO4 and K3[Co(NO2)6] are yellow colored compounds but Zn2[Fe(CN)6] is not yellow colored compound.
Which one has the highest boiling point?- a)He
- b)Ne
- c)Kr
- d)Xe
Correct answer is option 'D'. Can you explain this answer?
Which one has the highest boiling point?
a)
He
b)
Ne
c)
Kr
d)
Xe
|
|
Sanat Kumar answered |
As we go down the group the atomic size increases, melting point n Boiling point increases.
so,Here Xe is the very last element out there among the options given so the answer is Xe.
In the following circuit, the current through the resistor R (=2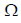 ) is I Amperes. The value of I is
) is I Amperes. The value of I is
Correct answer is '1'. Can you explain this answer?
In the following circuit, the current through the resistor R (=2 ) is I Amperes. The value of I is
) is I Amperes. The value of I is

|
Vikas Saini answered |
1ohm/2ohm and 2ohm/4ohm are balanced wheat stone bridge,so 8ohm R will be removed then after solvinge them 2ohm R will come.Then 6ohm/12ohm And 2ohm/4ohm are in balanced wheat stone bridge then 10ohm resistance will be removed.Reff comes 6.5 ohm.I=V/R=6.5/6.5=1ANS
From the following statement regarding H2O2, choose the incorrect statement- a)It can act only as an oxidizing agent
- b)It decomposes on exposure to light
- c)It has to be stored in plastic or wax lined glass bottles in dark.
- d)It has to be kept away from dust
Correct answer is option 'A'. Can you explain this answer?
From the following statement regarding H2O2, choose the incorrect statement
a)
It can act only as an oxidizing agent
b)
It decomposes on exposure to light
c)
It has to be stored in plastic or wax lined glass bottles in dark.
d)
It has to be kept away from dust
|
|
Lavanya Menon answered |
H2O2 can be reduced or oxidised. Hence, it can act as reducing as well as oxidising agent.
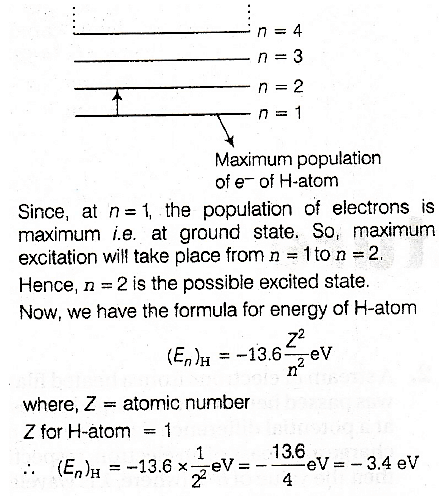
Section 1Q. No. 1 - 8 Carry 4 marks eachThe answer to each question is a SINGLE DIGIT INTEGER ranging from 0 to 9, both inclusive.Q.An electron in an excited state of Li2+ ion has angular momentum 3h/2π. The de Broglie wavelength of the
electron in this state is pπa0 (where a0 is the Bohr radius). The value of p is
Correct answer is '2'. Can you explain this answer?
Section 1
Q. No. 1 - 8 Carry 4 marks each
The answer to each question is a SINGLE DIGIT INTEGER ranging from 0 to 9, both inclusive.
Q.
An electron in an excited state of Li2+ ion has angular momentum 3h/2π. The de Broglie wavelength of the
electron in this state is pπa0 (where a0 is the Bohr radius). The value of p is
electron in this state is pπa0 (where a0 is the Bohr radius). The value of p is

|
Kumari Sakshi answered |

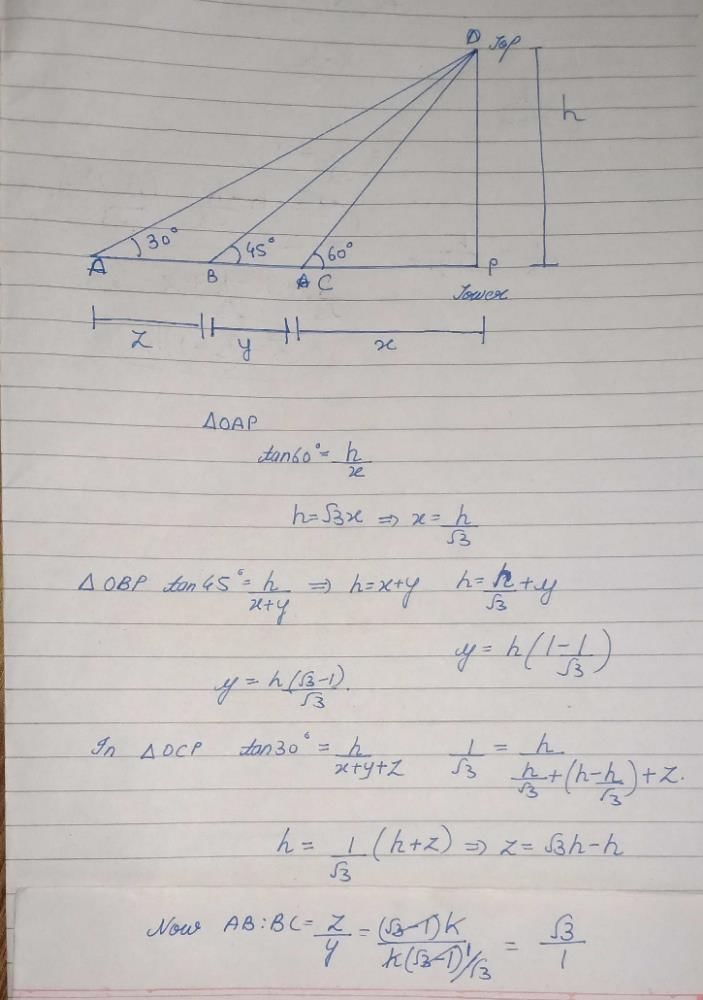
If the angles of elevation of the top of a tower from three collinear points A, B and C, on a line leading to the foot of the tower, are 30º, 45º and 60º respectively, then the ratio, AB : BC, is- a)√3 : 1
- b)√3 : √2
- c)1 : √3
- d)2 : 3
Correct answer is option 'A'. Can you explain this answer?
If the angles of elevation of the top of a tower from three collinear points A, B and C, on a line leading to the foot of the tower, are 30º, 45º and 60º respectively, then the ratio, AB : BC, is
a)
√3 : 1
b)
√3 : √2
c)
1 : √3
d)
2 : 3
|
|
Abhijeet Ingle answered |
In the long form of the periodic table, the valence shell electronic configuration of 5s2 5p4 corresponds to the element present in :- a)Group 16 and period 6
- b)Group 17 and period 5
- c)Group 16 and period 5
- d)Group 17 and period 6
Correct answer is option 'C'. Can you explain this answer?
In the long form of the periodic table, the valence shell electronic configuration of 5s2 5p4 corresponds to the element present in :
a)
Group 16 and period 6
b)
Group 17 and period 5
c)
Group 16 and period 5
d)
Group 17 and period 6
|
|
Shaurya Goel answered |
For finding the period and the group of an element whose valence orbital configuration is given-
(i) Check the principal 'n' value. The 'n' value gives the period in which the element is present.
Here 'n' is 5.
Therefore, the element is present in the 5th period.
(ii) Check the number of electrons in the valence shell.
No. of electrons in the valence shell = 4 + 2 = 6
Since the number of electrons is greater than 2, we must add 10 to get the group in which this element in present.
So, 10 + 6 = 16
Therefore, this element is present in the 16th group.
Match the catalysts to the correct processes :
Catalyst Process
a. TiCl3 (i) Wacker process
b. PdCl2 (ii) Ziegler-Natta polymerization
c. CuCl2 (iii) Contact process
d. V2O5 (iv) Deacon's process- a)a(iii), b(ii), c(iv), d(i)
- b)a(ii), b(i), c(iv), d(iii)
- c)a(ii), b(iii), c(iv), d(i)
- d)a(iii), b(i), c(ii), d(iv)
Correct answer is option 'B'. Can you explain this answer?
Match the catalysts to the correct processes :
Catalyst Process
a. TiCl3 (i) Wacker process
b. PdCl2 (ii) Ziegler-Natta polymerization
c. CuCl2 (iii) Contact process
d. V2O5 (iv) Deacon's process
Catalyst Process
a. TiCl3 (i) Wacker process
b. PdCl2 (ii) Ziegler-Natta polymerization
c. CuCl2 (iii) Contact process
d. V2O5 (iv) Deacon's process
a)
a(iii), b(ii), c(iv), d(i)
b)
a(ii), b(i), c(iv), d(iii)
c)
a(ii), b(iii), c(iv), d(i)
d)
a(iii), b(i), c(ii), d(iv)
|
|
Akash Kumar answered |
You see the given reactions are direct reactions from ncert.However i'll give u a short idea about that all.
TiCl3-- zeigler nata catalyst( used to prepare high demsity polythene also known as orlon)
PdCl3-- it is a reagent in wakers process.we prepare aldehyde in this process.
CuCl2-- it is a famous catalyst which is used to prepare chlorine gas.And the process is known as deccan process.
V2O5--we use this catalyst in contact process to convert sulphur dioxide into sulphur trioxide to prapare a final product i.e, sulphuric acid.
TiCl3-- zeigler nata catalyst( used to prepare high demsity polythene also known as orlon)
PdCl3-- it is a reagent in wakers process.we prepare aldehyde in this process.
CuCl2-- it is a famous catalyst which is used to prepare chlorine gas.And the process is known as deccan process.
V2O5--we use this catalyst in contact process to convert sulphur dioxide into sulphur trioxide to prapare a final product i.e, sulphuric acid.
An infinitely long uniform line charge distribution of charge per unit length λ lies parallel to the y-axis in the
y-z plane at z =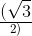 a (see figure). If the magnitude of the flux of the electric field through the rectangular
a (see figure). If the magnitude of the flux of the electric field through the rectangular
surface ABCD lying in the x-y plane with its center at the origin is 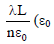 = permittivity of free space), then
= permittivity of free space), then
the value of n is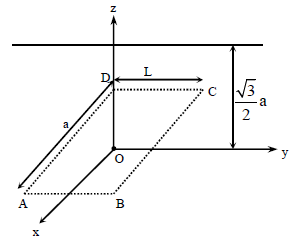
Correct answer is '6'. Can you explain this answer?
An infinitely long uniform line charge distribution of charge per unit length λ lies parallel to the y-axis in the
y-z plane at z =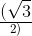 a (see figure). If the magnitude of the flux of the electric field through the rectangular
a (see figure). If the magnitude of the flux of the electric field through the rectangular
surface ABCD lying in the x-y plane with its center at the origin is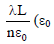 = permittivity of free space), then
= permittivity of free space), then
the value of n is
y-z plane at z =
surface ABCD lying in the x-y plane with its center at the origin is
the value of n is
|
|
Krishna Iyer answered |
In the context of the Hall-Heroult process for the extraction of Al, which of the following statement is false?- a)CO and CO2 are produced in this process
- b)Al2O3 is mixed with CaF2 which lowers the melting point of the mixture and brings conductivity
- c)Al3+ is reduced at the cathode to form Al
- d)Na3AlF6 serves as the electrolyte
Correct answer is option 'D'. Can you explain this answer?
In the context of the Hall-Heroult process for the extraction of Al, which of the following statement is false?
a)
CO and CO2 are produced in this process
b)
Al2O3 is mixed with CaF2 which lowers the melting point of the mixture and brings conductivity
c)
Al3+ is reduced at the cathode to form Al
d)
Na3AlF6 serves as the electrolyte
|
|
Chirag Verma answered |
In Hall-Heroult process Al2O3 (molten) is electrolyte.
Consider a hydrogen atom with its electron in the nth orbital. An electromagnetic radiation of wavelength
90 nm is used to ionize the atom. If the kinetic energy of the ejected electron is 10.4 eV, then the value of n
is (hc = 1242 eV nm)
Correct answer is '2'. Can you explain this answer?
Consider a hydrogen atom with its electron in the nth orbital. An electromagnetic radiation of wavelength
90 nm is used to ionize the atom. If the kinetic energy of the ejected electron is 10.4 eV, then the value of n
is (hc = 1242 eV nm)
90 nm is used to ionize the atom. If the kinetic energy of the ejected electron is 10.4 eV, then the value of n
is (hc = 1242 eV nm)
|
|
Rohit Jain answered |
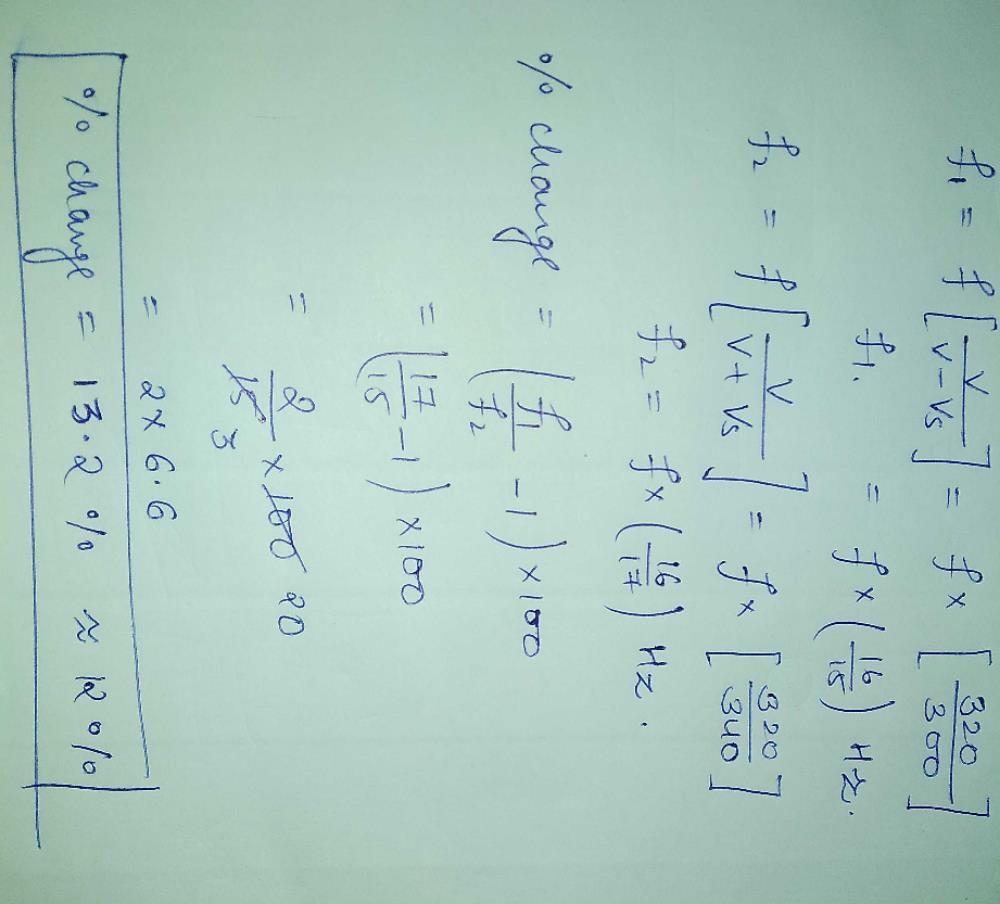
A train is moving on a straight track with speed 20 ms–1. It is blowing its whistle at the frequency of 1000 Hz. The percentage change in the frequency heard by a person standing near the track as the train passes him is (speed of sound = 320 ms–1) close to- a)6%
- b)12%
- c)18%
- d)24%
Correct answer is option 'B'. Can you explain this answer?
A train is moving on a straight track with speed 20 ms–1. It is blowing its whistle at the frequency of 1000 Hz. The percentage change in the frequency heard by a person standing near the track as the train passes him is (speed of sound = 320 ms–1) close to
a)
6%
b)
12%
c)
18%
d)
24%
|
|
Apoorva Kothari answered |
Let A and B be two sets containing four and two elements respectively. Then the number of subsets of the set A × B, each having at least three elements is- a)219
- b)256
- c)275
- d)510
Correct answer is option 'A'. Can you explain this answer?
Let A and B be two sets containing four and two elements respectively. Then the number of subsets of the set A × B, each having at least three elements is
a)
219
b)
256
c)
275
d)
510
|
|
Jatin Kulkarni answered |
n(A) = 4, n(B) = 2
n(A × B) = 8
Required numbers = 8C3 + 8C4 + ...... + 8C8
= 28 – (8C0 + 8C1 + 8C2)
= 256 – 37
= 219
n(A × B) = 8
Required numbers = 8C3 + 8C4 + ...... + 8C8
= 28 – (8C0 + 8C1 + 8C2)
= 256 – 37
= 219
The area (in sq. units) of the quadrilateral formed by the tangents at the end points of the latera recta to the ellipse 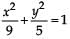 , is
, is- a)27/4
- b)18
- c)27/2
- d)27
Correct answer is option 'D'. Can you explain this answer?
The area (in sq. units) of the quadrilateral formed by the tangents at the end points of the latera recta to the ellipse 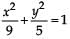 , is
, is
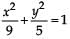 , is
, isa)
27/4
b)
18
c)
27/2
d)
27
|
|
Aditya Oza answered |
. I can help you out with the objective approach . Once draw the diagram and you would find that the rectangle formed by the latus rectum with each other is fully enclosed by the asked quad. so obviously the req quad. will have larger area . find area of the rectangle formed by latus rectums and you would find it more than three options leaving out the correct option . Hope this would help . Ask me if you want the subjective approach too .
Have a nice day :)
Have a nice day :)
When O2 is adsorbed on a metallic surface, electron transfer occurs from the metal to O2. The TRUE
statement(s) regarding this adsorption is(are)- a)O2 is physisorbed
- b)heat is released
- c)occupancy of π*2p of O2 is increased
- d)bond length of O2 is increased
Correct answer is option 'B,C,D'. Can you explain this answer?
When O2 is adsorbed on a metallic surface, electron transfer occurs from the metal to O2. The TRUE
statement(s) regarding this adsorption is(are)
statement(s) regarding this adsorption is(are)
a)
O2 is physisorbed
b)
heat is released
c)
occupancy of π*2p of O2 is increased
d)
bond length of O2 is increased
|
|
Disha Khanna answered |
* Adsorption of O2 on metal surface is exothermic.
* During electron transfer from metal to O2 electron occupies π*2p orbital of O2.
* Due to electron transfer to O2 the bond order of O2 decreases hence bond length increases
* During electron transfer from metal to O2 electron occupies π*2p orbital of O2.
* Due to electron transfer to O2 the bond order of O2 decreases hence bond length increases
The least value of the product xyz for which the determinant 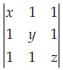 is non-negative, is :
is non-negative, is :- a)- 2√2
- b)- 16√2
- c)- 8
- d)- 1
Correct answer is option 'C'. Can you explain this answer?
The least value of the product xyz for which the determinant 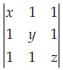 is non-negative, is :
is non-negative, is :
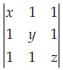 is non-negative, is :
is non-negative, is :a)
- 2√2
b)
- 16√2
c)
- 8
d)
- 1

|
Saanket Mondal answered |
On solving, xyz-x-y-z+2 is value of the determinant. It is non negative also. Thus xyz-x-y-z+2=0(least non negative)
So xyz-(x+y+z)=-2
By hit and trial method, we can see x=y=z=-2 satisfies above equation. So xyz= -2×-2×-2=-8
So xyz-(x+y+z)=-2
By hit and trial method, we can see x=y=z=-2 satisfies above equation. So xyz= -2×-2×-2=-8
The pair(s) of ions where BOTH the ions are precipitated upon passing H2S gas in presence of dilute HCl,
is(are)- a)Ba2+, Zn2+
- b)Bi3+, Fe3+
- c)Cu2+, Pb2+
- d)Hg2+, Bi3+
Correct answer is option 'C,D'. Can you explain this answer?
The pair(s) of ions where BOTH the ions are precipitated upon passing H2S gas in presence of dilute HCl,
is(are)
is(are)
a)
Ba2+, Zn2+
b)
Bi3+, Fe3+
c)
Cu2+, Pb2+
d)
Hg2+, Bi3+
|
|
Nitya Sarkar answered |
Cu2+ , Pb2+ , Hg2+ , Bi3+ give ppt with H2S in presence of dilute HCl.
Let n be the number of ways in which 5 boys and 5 girls can stand in a queue in such a way that all the girls
stand consecutively in the queue. Let m be the number of ways in which 5 boys and 5 girls can stand in a
queue in such a way that exactly four girls stand consecutively in the queue. Then the value of m/n is
Correct answer is '5'. Can you explain this answer?
Let n be the number of ways in which 5 boys and 5 girls can stand in a queue in such a way that all the girls
stand consecutively in the queue. Let m be the number of ways in which 5 boys and 5 girls can stand in a
queue in such a way that exactly four girls stand consecutively in the queue. Then the value of m/n is
stand consecutively in the queue. Let m be the number of ways in which 5 boys and 5 girls can stand in a
queue in such a way that exactly four girls stand consecutively in the queue. Then the value of m/n is
|
|
Anshul Iyer answered |
(4 out of 5 girls together arranged with others – number of cases all 5 girls are together)
The correct statement(s) regarding, (i) HClO, (ii) HClO2, (iii) HClO3 and (iv) HClO4, is (are)- a)The number of Cl = O bonds in (ii) and (iii) together is two
- b)The number of lone pairs of electrons on Cl in (ii) and (iii) together is three
- c)The hybridization of Cl in (iv) is sp3
- d)Amongst (i) to (iv), the strongest acid is (i)
Correct answer is option 'B,C'. Can you explain this answer?
The correct statement(s) regarding, (i) HClO, (ii) HClO2, (iii) HClO3 and (iv) HClO4, is (are)
a)
The number of Cl = O bonds in (ii) and (iii) together is two
b)
The number of lone pairs of electrons on Cl in (ii) and (iii) together is three
c)
The hybridization of Cl in (iv) is sp3
d)
Amongst (i) to (iv), the strongest acid is (i)
|
|
Krishna Iyer answered |
For the octahedral complexes of Fe3+ in SCN– (thiocyanato-S) and in CN– ligand environments, the
difference between the spin-only magnetic moments in Bohr magnetons (When approximated to the nearest
integer) is
[Atomic number of Fe = 26]
Correct answer is '4'. Can you explain this answer?
For the octahedral complexes of Fe3+ in SCN– (thiocyanato-S) and in CN– ligand environments, the
difference between the spin-only magnetic moments in Bohr magnetons (When approximated to the nearest
integer) is
[Atomic number of Fe = 26]
difference between the spin-only magnetic moments in Bohr magnetons (When approximated to the nearest
integer) is
[Atomic number of Fe = 26]
|
|
Mayank Saha answered |
A block of mass m = 0.1 kg is connected to a spring of unknown spring constant k. It is compressed to a distance x from its equilibrium position and released from rest. After approaching half the distance (x/2) from equilibrium position, it hits another block and comes to rest momentarily, while the other block moves with a velocity 3 ms-1 . The total initial energy of the spring is :- a)1.5 J
- b)0.6 J
- c)0.3 J
- d)0.8 J
Correct answer is option 'B'. Can you explain this answer?
A block of mass m = 0.1 kg is connected to a spring of unknown spring constant k. It is compressed to a distance x from its equilibrium position and released from rest. After approaching half the distance (x/2) from equilibrium position, it hits another block and comes to rest momentarily, while the other block moves with a velocity 3 ms-1 . The total initial energy of the spring is :
a)
1.5 J
b)
0.6 J
c)
0.3 J
d)
0.8 J
|
|
Maya Chopra answered |
Given:
- Mass of the block, m = 0.1 kg
- Distance the spring is compressed, x
- Velocity of the other block after collision, v = 3 m/s
To find:
Total initial energy of the spring
Assumptions:
- The collision between the two blocks is perfectly elastic, i.e., no energy is lost during the collision.
- There is no external force acting on the system except gravity.
Analysis:
1. When the block is compressed to a distance x from its equilibrium position, it possesses potential energy due to the compressed spring.
2. The potential energy stored in a spring is given by the formula: PE = (1/2)kx^2, where k is the spring constant.
3. When the block is released, it starts oscillating back and forth around its equilibrium position.
4. At the midpoint of its oscillation, i.e., when it is at a distance of x/2 from the equilibrium position, it comes to rest momentarily.
5. At this point, all its potential energy has been converted into kinetic energy.
6. The kinetic energy of an object is given by the formula: KE = (1/2)mv^2, where m is the mass of the object and v is its velocity.
7. Since the block comes to rest momentarily, its velocity at that point is zero. Therefore, the kinetic energy at that point is also zero.
8. At this point, the other block, with a velocity of 3 m/s, collides with the first block.
9. Since the collision is perfectly elastic, the total kinetic energy of the system remains conserved.
10. After the collision, the first block comes to rest and its kinetic energy becomes zero again.
11. The total initial energy of the spring is equal to the sum of the potential energy and kinetic energy of the first block just before the collision.
Solution:
1. The potential energy of the spring at distance x is given by PE = (1/2)kx^2.
2. At the midpoint of the oscillation, the block comes to rest and all its potential energy is converted into kinetic energy. Therefore, (1/2)k(x/2)^2 = (1/2)mv^2.
3. Solving this equation for k, we get k = (m*v^2)/(x^2/4) = 4m*v^2/x^2.
4. The total initial energy of the spring is given by the sum of potential energy and kinetic energy just before the collision.
Total initial energy = PE + KE = (1/2)kx^2 + (1/2)mv^2.
5. Substituting the value of k, we get Total initial energy = (1/2)(4m*v^2/x^2)*x^2 + (1/2)mv^2 = 2mv^2 + (1/2)mv^2 = 5/2mv^2.
6. Substituting the given values, Total initial energy = (5/2)*(0.1)*(3)^2 = 0.6 J.
Therefore, the correct answer is option 'B': 0.6 J.
- Mass of the block, m = 0.1 kg
- Distance the spring is compressed, x
- Velocity of the other block after collision, v = 3 m/s
To find:
Total initial energy of the spring
Assumptions:
- The collision between the two blocks is perfectly elastic, i.e., no energy is lost during the collision.
- There is no external force acting on the system except gravity.
Analysis:
1. When the block is compressed to a distance x from its equilibrium position, it possesses potential energy due to the compressed spring.
2. The potential energy stored in a spring is given by the formula: PE = (1/2)kx^2, where k is the spring constant.
3. When the block is released, it starts oscillating back and forth around its equilibrium position.
4. At the midpoint of its oscillation, i.e., when it is at a distance of x/2 from the equilibrium position, it comes to rest momentarily.
5. At this point, all its potential energy has been converted into kinetic energy.
6. The kinetic energy of an object is given by the formula: KE = (1/2)mv^2, where m is the mass of the object and v is its velocity.
7. Since the block comes to rest momentarily, its velocity at that point is zero. Therefore, the kinetic energy at that point is also zero.
8. At this point, the other block, with a velocity of 3 m/s, collides with the first block.
9. Since the collision is perfectly elastic, the total kinetic energy of the system remains conserved.
10. After the collision, the first block comes to rest and its kinetic energy becomes zero again.
11. The total initial energy of the spring is equal to the sum of the potential energy and kinetic energy of the first block just before the collision.
Solution:
1. The potential energy of the spring at distance x is given by PE = (1/2)kx^2.
2. At the midpoint of the oscillation, the block comes to rest and all its potential energy is converted into kinetic energy. Therefore, (1/2)k(x/2)^2 = (1/2)mv^2.
3. Solving this equation for k, we get k = (m*v^2)/(x^2/4) = 4m*v^2/x^2.
4. The total initial energy of the spring is given by the sum of potential energy and kinetic energy just before the collision.
Total initial energy = PE + KE = (1/2)kx^2 + (1/2)mv^2.
5. Substituting the value of k, we get Total initial energy = (1/2)(4m*v^2/x^2)*x^2 + (1/2)mv^2 = 2mv^2 + (1/2)mv^2 = 5/2mv^2.
6. Substituting the given values, Total initial energy = (5/2)*(0.1)*(3)^2 = 0.6 J.
Therefore, the correct answer is option 'B': 0.6 J.
If y + 3x = 0 is the equation of a chord of the circle, x2 + y2 - 30x = 0, then the equation of the circle with this chord as diameter is :- a)x2 + y2 + 3x + 9y = 0
- b)x2 + y2 - 3x + 9y = 0
- c)x2 + y2 - 3x - 9y = 0
- d)x2 + y2 + 3x - 9y = 0
Correct answer is option 'B'. Can you explain this answer?
If y + 3x = 0 is the equation of a chord of the circle, x2 + y2 - 30x = 0, then the equation of the circle with this chord as diameter is :
a)
x2 + y2 + 3x + 9y = 0
b)
x2 + y2 - 3x + 9y = 0
c)
x2 + y2 - 3x - 9y = 0
d)
x2 + y2 + 3x - 9y = 0
|
|
Ambrishi answered |
X^2+y^2-3x+9y=0
Chapter doubts & questions for JEE Main & Advanced (2015) - JEE Main & Advanced Mock Test Series 2025 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of JEE Main & Advanced (2015) - JEE Main & Advanced Mock Test Series 2025 in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup
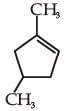
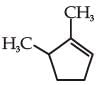
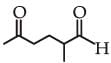
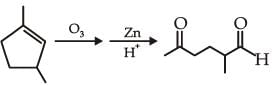

 and the
and the
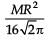
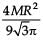
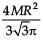
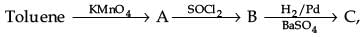 the product C is
the product C is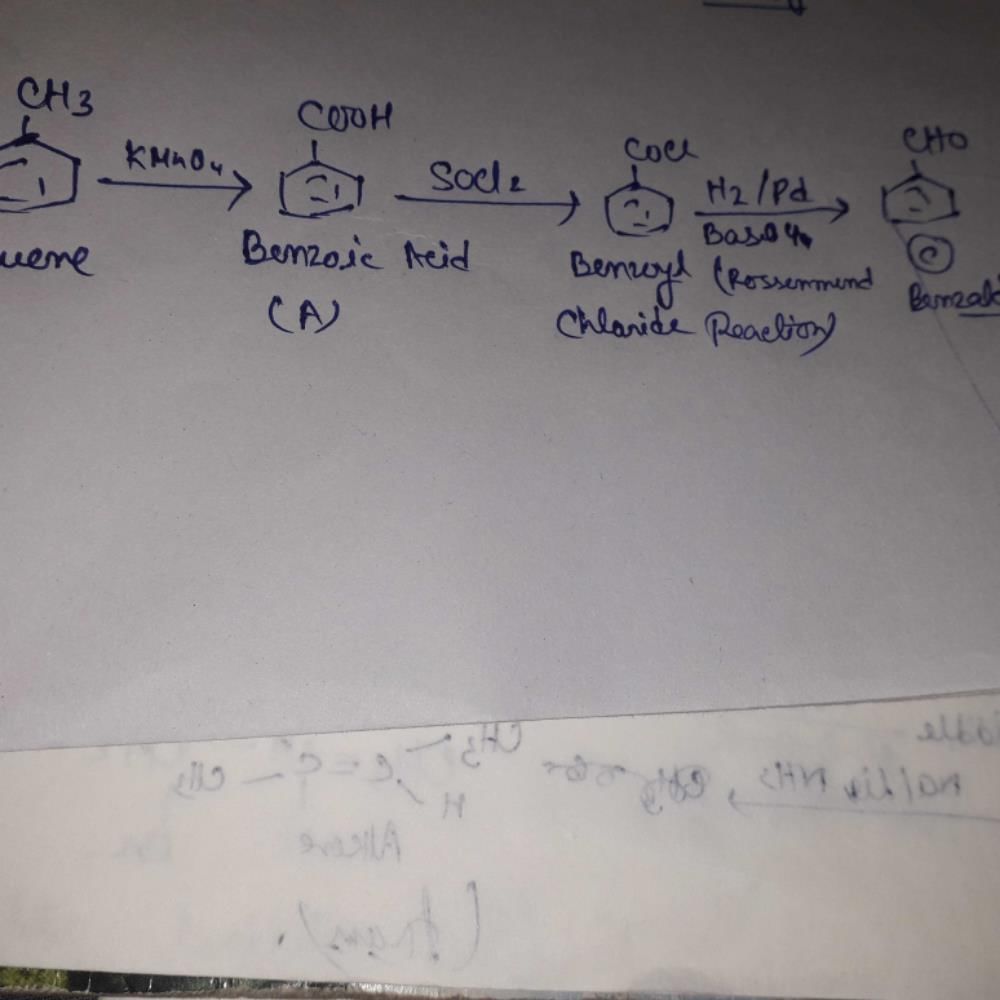

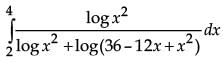 is equal to
is equal to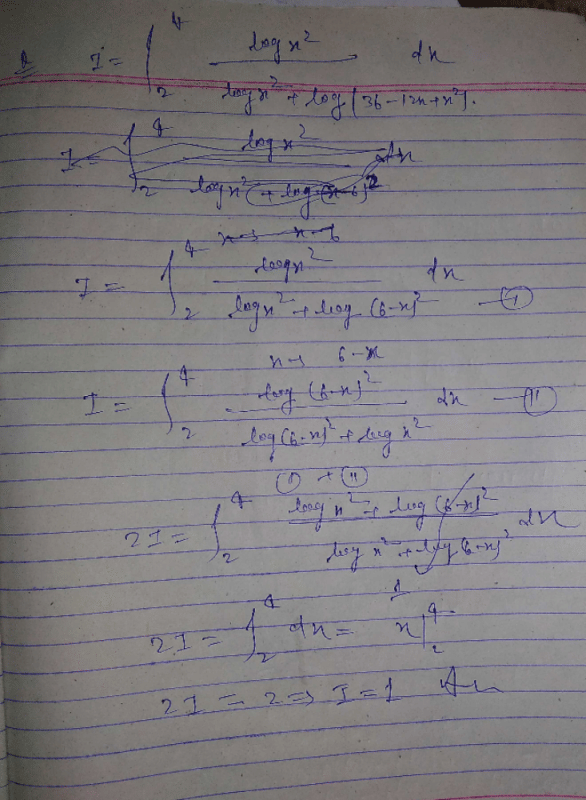
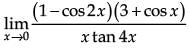 is equal to
is equal to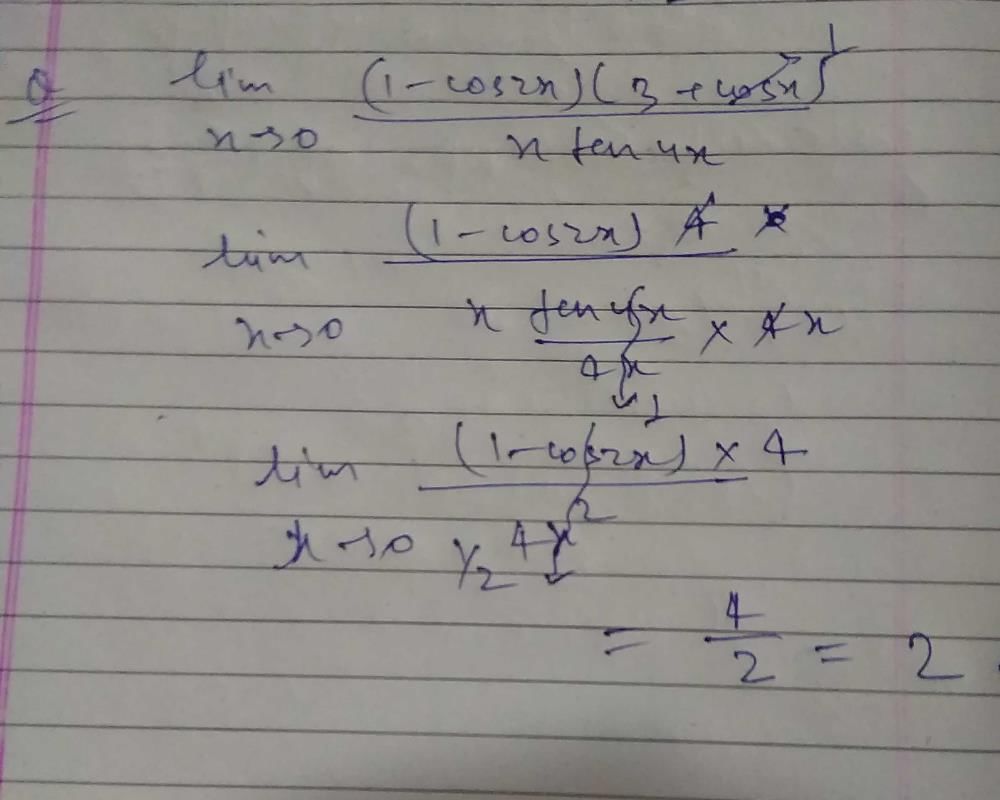
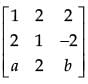 is a matrix satisfying the equation AAT = 9I, where I is 3 × 3 identity matrix, then the ordered pair (a, b) is equal to
is a matrix satisfying the equation AAT = 9I, where I is 3 × 3 identity matrix, then the ordered pair (a, b) is equal to








