All Exams >
Class 11 >
Chemistry Class 11 >
All Questions
All questions of Classification of Elements & Periodicity in Properties for Class 11 Exam
Lothar Meyer proposed that on arranging the elements in order of increasing atomic weights; similarities appear in which type of properties?- a)Only physical properties
- b)Only chemical properties
- c)In both physical and chemical properties
- d)thermodynamic properties
Correct answer is option 'C'. Can you explain this answer?
Lothar Meyer proposed that on arranging the elements in order of increasing atomic weights; similarities appear in which type of properties?
a)
Only physical properties
b)
Only chemical properties
c)
In both physical and chemical properties
d)
thermodynamic properties
|
|
Anoushka Yadav answered |
Lothar Meyer proposed the periodic table in 1864. He arranged the elements in order of increasing atomic weights. He noticed that similarities appeared in both physical and chemical properties of the elements. The correct answer is option 'C' which means similarities appear in both physical and chemical properties.
Explanation:
The periodic table arranges the elements in a way that helps in understanding their properties. The modern periodic table is based on the electronic configuration of the elements. But the original periodic table was based on the atomic weight of the elements. Lothar Meyer was the first to realize that there is a periodicity in the properties of the elements when they are arranged in order of increasing atomic weights.
Physical properties are those that can be observed or measured without changing the chemical composition of the substance. Some examples of physical properties are:
- Melting point
- Boiling point
- Density
- Electrical conductivity
- Thermal conductivity
- Atomic radius
- Ionic radius
- Electronegativity
Chemical properties are those that can be observed during a chemical reaction. Some examples of chemical properties are:
- Reactivity with acids
- Reactivity with oxygen
- Reactivity with water
- Reduction potential
- Oxidation potential
Meyer observed that when the elements are arranged in order of increasing atomic weights, there is a periodicity in their physical and chemical properties. This means that elements with similar atomic weights have similar physical and chemical properties. For example, lithium, sodium, and potassium have similar physical and chemical properties because they all belong to the same group and have similar atomic weights.
Conclusion:
Lothar Meyer proposed the periodic table in 1864. He arranged the elements in order of increasing atomic weights. He noticed that similarities appeared in both physical and chemical properties of the elements. When the elements are arranged in order of increasing atomic weights, there is a periodicity in their physical and chemical properties.
Explanation:
The periodic table arranges the elements in a way that helps in understanding their properties. The modern periodic table is based on the electronic configuration of the elements. But the original periodic table was based on the atomic weight of the elements. Lothar Meyer was the first to realize that there is a periodicity in the properties of the elements when they are arranged in order of increasing atomic weights.
Physical properties are those that can be observed or measured without changing the chemical composition of the substance. Some examples of physical properties are:
- Melting point
- Boiling point
- Density
- Electrical conductivity
- Thermal conductivity
- Atomic radius
- Ionic radius
- Electronegativity
Chemical properties are those that can be observed during a chemical reaction. Some examples of chemical properties are:
- Reactivity with acids
- Reactivity with oxygen
- Reactivity with water
- Reduction potential
- Oxidation potential
Meyer observed that when the elements are arranged in order of increasing atomic weights, there is a periodicity in their physical and chemical properties. This means that elements with similar atomic weights have similar physical and chemical properties. For example, lithium, sodium, and potassium have similar physical and chemical properties because they all belong to the same group and have similar atomic weights.
Conclusion:
Lothar Meyer proposed the periodic table in 1864. He arranged the elements in order of increasing atomic weights. He noticed that similarities appeared in both physical and chemical properties of the elements. When the elements are arranged in order of increasing atomic weights, there is a periodicity in their physical and chemical properties.
Horizontal rows in the periodic table are called:- a)Cell
- b)Table
- c)Groups
- d)Periods
Correct answer is option 'D'. Can you explain this answer?
Horizontal rows in the periodic table are called:
a)
Cell
b)
Table
c)
Groups
d)
Periods
|
|
Rohan Singh answered |
The Periodic Table: Families and Periods. In the periodic table of elements, there are seven horizontal rows of elements called periods. The vertical columns of elements are called groups, or families.
Can you explain the answer of this question below:In the modern periodic table, which period contains 32 elements?
- A:
Sixth
- B:
First
- C:
Seventh
- D:
Second
The answer is a.
In the modern periodic table, which period contains 32 elements?
Sixth
First
Seventh
Second
|
|
Preeti Iyer answered |
The answer is c.
The total number of electrons that can be accommodated in seventh period are 2 ( in 7s) + 14(in 5f) + 10(in 6d )+ 6(in 7p) = 32. The maximum number of elements present in it is 32.
Among the following statements the one that is not true about Mendeleev’s Periodic Table is:- a)Elements of group 7 and 8 were arranged on the basis of equivalent weights
- b)Some elements in the same group differ in their properties
- c)Elements were arranged on the basis of atomic weights
- d)The position of hydrogen was not justified
Correct answer is option 'A'. Can you explain this answer?
Among the following statements the one that is not true about Mendeleev’s Periodic Table is:
a)
Elements of group 7 and 8 were arranged on the basis of equivalent weights
b)
Some elements in the same group differ in their properties
c)
Elements were arranged on the basis of atomic weights
d)
The position of hydrogen was not justified
|
|
Om Desai answered |
In Mendeleev’s periodic table, elements were arranged on the basis of atomic weights.
An element with atomic number will form a basic oxide________ - a)7
- b)17
- c)14
- d)11
Correct answer is option 'D'. Can you explain this answer?
An element with atomic number will form a basic oxide________
a)
7
b)
17
c)
14
d)
11

|
EduRev NEET answered |
Sodium forms a basic oxide because it is a metal. Metals generally form basic oxides because they tend to donate electrons to oxygen atoms. In the case of sodium, when it reacts with oxygen, it forms sodium oxide, in which each sodium atom donates one electron to oxygen. This results in the formation of sodium ions (Na+) and oxide ions (O2-), which can combine to form the ionic compound sodium oxide. Sodium oxide is basic because it can react with water to form a solution of sodium hydroxide, a strong base.
Eka silicon predicted by Mendeleev is which element:- a)Germanium
- b)Aluminium
- c)Gallium
- d)Sodium
Correct answer is option 'A'. Can you explain this answer?
Eka silicon predicted by Mendeleev is which element:
a)
Germanium
b)
Aluminium
c)
Gallium
d)
Sodium
|
|
Suresh Iyer answered |
Mendeleev predicted the existence of 'eka-silicon', which would fit into a gap next to silicon. The element germanium was discovered later. Its properties were found to be similar to the predicted ones and confirmed Mendeleev's periodic table.
The lanthanide series starts with:
- a)Thorium
- b)Lutetium
- c)Lawrencium
- d)cerium
Correct answer is option 'D'. Can you explain this answer?
The lanthanide series starts with:
a)
Thorium
b)
Lutetium
c)
Lawrencium
d)
cerium
|
|
Suresh Reddy answered |
The lanthanide series starts from cerium and ends in lutetium
Na+ is smaller than Na atom because:- a)The effective nuclear charge is greater in case of sodium ion.
- b)Nucleus in each case contains different nucleons.
- c)Na+ is more stable than in Na atom.
- d)Sodium atom has one electron lesser than sodium ion.
Correct answer is option 'A'. Can you explain this answer?
Na+ is smaller than Na atom because:
a)
The effective nuclear charge is greater in case of sodium ion.
b)
Nucleus in each case contains different nucleons.
c)
Na+ is more stable than in Na atom.
d)
Sodium atom has one electron lesser than sodium ion.
|
|
Geetika Shah answered |
According to the electronic configuration of sodium(Na),it has 1 valence electron(2,8,1).So it loses one electron to form a cation and becomes sodium cation (Na+).As it loses one electron ,the outermost shell has 0 electrons (i.e it gets eliminated ) and Na+ has only 2 shells. Hence, it lost one of its shell, the sodium ion is smaller than the sodium atom.
What is the relationship between the number of elements in each period and the number of atomic orbitals available in the energy level that is being filled?- a)Number of elements is twice the number of atomic orbitals.
- b)Number of elements is three times the number of atomic orbitals.
- c)Number of elements is half the number of atomic orbitals.
- d)Number of elements is same as the number of atomic orbitals.
Correct answer is 'A'. Can you explain this answer?
What is the relationship between the number of elements in each period and the number of atomic orbitals available in the energy level that is being filled?
a)
Number of elements is twice the number of atomic orbitals.
b)
Number of elements is three times the number of atomic orbitals.
c)
Number of elements is half the number of atomic orbitals.
d)
Number of elements is same as the number of atomic orbitals.
|
|
Krishna Iyer answered |
The number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled.
Let's understand it by taking the elements of the 4th period.
In the fourth period, there are 18 elements. The types of subshells used in the 4th period are s, p, and d.
Number of orbitals of s subshell = 1
Number of orbitals of p subshell = 3
Number of orbitals of d subshell = 5
Total number of orbitals = 9
The total number of elements is 18 i.e. twice the total number of orbitals.
In the fourth period, there are 18 elements. The types of subshells used in the 4th period are s, p, and d.
Number of orbitals of s subshell = 1
Number of orbitals of p subshell = 3
Number of orbitals of d subshell = 5
Total number of orbitals = 9
The total number of elements is 18 i.e. twice the total number of orbitals.
Which scientist proposed that atomic number is more fundamental property of an element than its atomic mass?- a)Newlands
- b)Lothar Meyer
- c)Henry Moseley
- d)Meyer
Correct answer is option 'C'. Can you explain this answer?
Which scientist proposed that atomic number is more fundamental property of an element than its atomic mass?
a)
Newlands
b)
Lothar Meyer
c)
Henry Moseley
d)
Meyer
|
|
Pooja Shah answered |
The scientist who first of all showed that the atomic number is more fundamental property of an element than its atomic mass was Henry Moseley.
Johann Dobereiner classified elements in group of three elements called as- a)Trinity
- b)Trials
- c)Triads
- d)Diads
Correct answer is option 'C'. Can you explain this answer?
Johann Dobereiner classified elements in group of three elements called as
a)
Trinity
b)
Trials
c)
Triads
d)
Diads
|
|
Om Desai answered |
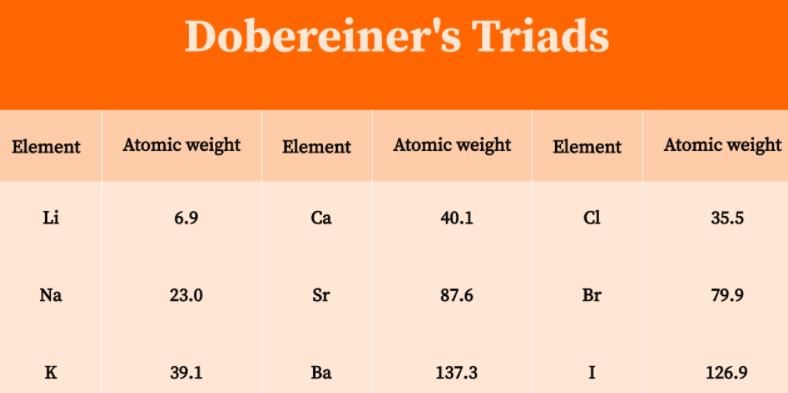
In 1829, Johann Dobereiner, a German scientist made some groups of three elements each and called them triads.
He observed that the atomic mass of the middle element of a triad was nearly equal to the arithmetic mean of the atomic masses of the other two elements. All three elements of a triad were similar in their properties.
The electronegativity of following elements increases in the order of?- a)Si, P, C, N
- b)P, Si, N, C
- c)C, N, Si, P
- d)N, Si, C, P
Correct answer is option 'A'. Can you explain this answer?
The electronegativity of following elements increases in the order of?
a)
Si, P, C, N
b)
P, Si, N, C
c)
C, N, Si, P
d)
N, Si, C, P
|
|
Pooja Shah answered |
Si,P,C,N
Electronegativity increases along a period and decreases down the group.Nitrogen is expected to have maximum electronegativity.
According to Dobereiner’s law of triads the number of elements present in each group is:
- a)2
- b)4
- c)5
- d)3
Correct answer is option 'D'. Can you explain this answer?
According to Dobereiner’s law of triads the number of elements present in each group is:
a)
2
b)
4
c)
5
d)
3
|
|
Geetika Shah answered |
- According to Dobereiner's law of triads each triad contains three elements.
- He also noticed that the middle element of each of the triads had an atomic weight about halfway between the atomic weights of the other two.
Eka aluminium predicted by Mendeleev is which element?- a)Germanium
- b)Magnesium
- c)Gallium
- d)Sodium
Correct answer is option 'C'. Can you explain this answer?
Eka aluminium predicted by Mendeleev is which element?
a)
Germanium
b)
Magnesium
c)
Gallium
d)
Sodium
|
|
Gaurav Kumar answered |
Eka aluminium predicted by Mendeleev is Gallium. Eka-aluminium and gallium are the two names of the same element as Eka -Aluminum has almost exactly the same properties as the actual properties of the gallium element.
Among the elements with atomic numbers 9, 12, 16 and 36 which is highly electropositive?- a)Element with atomic number 9
- b)Element \vith atomic number 12
- c)Element with atomic number 16
- d)Element with atomic number 36
Correct answer is option 'B'. Can you explain this answer?
Among the elements with atomic numbers 9, 12, 16 and 36 which is highly electropositive?
a)
Element with atomic number 9
b)
Element \vith atomic number 12
c)
Element with atomic number 16
d)
Element with atomic number 36
|
|
Pooja Deshpande answered |
Introduction:
In order to determine which element is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36, we need to understand the concept of electronegativity and how it relates to electropositivity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Electropositivity, on the other hand, is the tendency of an atom to lose electrons and form positive ions. Therefore, an element with low electronegativity and high electropositivity will readily lose electrons and form positive ions.
Explanation:
Let's analyze each element and its electropositivity based on their atomic numbers:
Element with atomic number 9 (Fluorine):
Fluorine is a halogen and has a high electronegativity. It readily gains an electron to achieve a stable electron configuration. Therefore, it is not highly electropositive.
Element with atomic number 12 (Magnesium):
Magnesium is an alkaline earth metal and has a low electronegativity. It readily loses its two valence electrons to achieve a stable electron configuration. Therefore, it is highly electropositive.
Element with atomic number 16 (Sulfur):
Sulfur is a nonmetal and has a moderate electronegativity. It can gain or lose electrons depending on the reaction conditions. However, it is not highly electropositive as it tends to gain electrons to achieve a stable electron configuration.
Element with atomic number 36 (Krypton):
Krypton is a noble gas and has a very high electronegativity. It has a stable electron configuration and tends not to lose or gain electrons. Therefore, it is not highly electropositive.
Conclusion:
Based on the analysis, the element with atomic number 12 (Magnesium) is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36. It has a low electronegativity and readily loses its two valence electrons to form a stable 2+ ion.
In order to determine which element is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36, we need to understand the concept of electronegativity and how it relates to electropositivity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Electropositivity, on the other hand, is the tendency of an atom to lose electrons and form positive ions. Therefore, an element with low electronegativity and high electropositivity will readily lose electrons and form positive ions.
Explanation:
Let's analyze each element and its electropositivity based on their atomic numbers:
Element with atomic number 9 (Fluorine):
Fluorine is a halogen and has a high electronegativity. It readily gains an electron to achieve a stable electron configuration. Therefore, it is not highly electropositive.
Element with atomic number 12 (Magnesium):
Magnesium is an alkaline earth metal and has a low electronegativity. It readily loses its two valence electrons to achieve a stable electron configuration. Therefore, it is highly electropositive.
Element with atomic number 16 (Sulfur):
Sulfur is a nonmetal and has a moderate electronegativity. It can gain or lose electrons depending on the reaction conditions. However, it is not highly electropositive as it tends to gain electrons to achieve a stable electron configuration.
Element with atomic number 36 (Krypton):
Krypton is a noble gas and has a very high electronegativity. It has a stable electron configuration and tends not to lose or gain electrons. Therefore, it is not highly electropositive.
Conclusion:
Based on the analysis, the element with atomic number 12 (Magnesium) is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36. It has a low electronegativity and readily loses its two valence electrons to form a stable 2+ ion.
Moseley performed experiments and studied the frequencies of which radiations emitted from the elements?- a)Gamma rays
- b)X-rays
- c)UV rays
- d)Infra red rays
Correct answer is option 'B'. Can you explain this answer?
Moseley performed experiments and studied the frequencies of which radiations emitted from the elements?
a)
Gamma rays
b)
X-rays
c)
UV rays
d)
Infra red rays
|
|
Naina Bansal answered |
Moseley performed experiments and studied the frequencies of the X-rays emitted from the elements.
Elements in the same group have same:- a)Number of valence electrons
- b)Density
- c)Atomic radius
- d)Nuclear charge
Correct answer is option 'A'. Can you explain this answer?
Elements in the same group have same:
a)
Number of valence electrons
b)
Density
c)
Atomic radius
d)
Nuclear charge
|
|
Lavanya Menon answered |
- Elements in the same group have the same number of valence electrons in their outermost shell, hence, have similar properties.
- Elements in the same period don't have same number of valence electrons, hence, have different properties. But, elements in the same period have same number of shells.
The first period has how many orbitals?- a)1
- b)3
- c)2
- d)4
Correct answer is option 'A'. Can you explain this answer?
The first period has how many orbitals?
a)
1
b)
3
c)
2
d)
4
|
|
Raghav Bansal answered |
1st period contains only 2 elements hydrogen and helium having atomic no. 1 and 2 respectively.
The electronic configuration of hydrogen is 1s1 while that of helium is 1s2. Both contain only s subshell. S subshell that only contains 1 orbital i.e. z.
Thus, 1st period has 1 orbital.
The symbol for element with atomic number 111 and name Unununnium is- a)Uun
- b)Uuu
- c)UUU
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
The symbol for element with atomic number 111 and name Unununnium is
a)
Uun
b)
Uuu
c)
UUU
d)
None of the above
|
|
Gaurav Kumar answered |
- The element was to be called unununium (with the corresponding symbol of Uuu),a systematic element name as a placeholder, until the element was discovered (and the discovery then confirmed) and a permanent name was decided on.
- Although widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, the recommendations were mostly ignored among scientists in the field, who called it element 111, with the symbol of E111, (111) or even simply 111.
The element with lowest ionization energy among the following is:- a)Li
- b)Na
- c)Rb
- d)K
Correct answer is option 'C'. Can you explain this answer?
The element with lowest ionization energy among the following is:
a)
Li
b)
Na
c)
Rb
d)
K
|
|
Gaurav Kumar answered |
When we go down in a group shell increase and size increase and due to large size of atom electron can easily be removed. Thats option is C.
Identify the smallest ion among the following:- a)Na+
- b)Mg2+
- c)K+
- d)Al3+
Correct answer is option 'D'. Can you explain this answer?
Identify the smallest ion among the following:
a)
Na+
b)
Mg2+
c)
K+
d)
Al3+
|
|
Raghav Bansal answered |
Al3+ because they all have the same number of electrons, however Al3+ has the most protons and therefore greatest nuclear charge. In Al3+, the same number of electrons are being attracted by more protons in the nucleus, so the electrons are being pulled closer to the nucleus, reducing the size of the ionic radius.
Which of the following elements will have the largest atomic radii?- a)Sr
- b)Al
- c)Li
- d)Ca
Correct answer is option 'A'. Can you explain this answer?
Which of the following elements will have the largest atomic radii?
a)
Sr
b)
Al
c)
Li
d)
Ca
|
|
Pooja Shah answered |
Strontium has the highest atomic radii because it on the group atomic radius increases also it belongs to 4D so if there is doubt of Lanthanide contraction then the size of 4D is largest followed by 3D and 5D has the smallest size.
Among the following elements element with highest ionisation enthalpy is:- a)N
- b)C
- c)O
- d)B
Correct answer is option 'A'. Can you explain this answer?
Among the following elements element with highest ionisation enthalpy is:
a)
N
b)
C
c)
O
d)
B
|
|
Pooja Shah answered |
Among the given molecules, N2 has highest ionization enthalpy.The reason behind is the stability of half-filled orbitals which makes it more stable than other elements in the given option list.
The basis of long form of periodic table is:- a)Mass number
- b)Atomic weight
- c)Atomic number
- d)Ionic radius
Correct answer is option 'C'. Can you explain this answer?
The basis of long form of periodic table is:
a)
Mass number
b)
Atomic weight
c)
Atomic number
d)
Ionic radius
|
|
Nandini Patel answered |
In long form of periodic table elements are arranged in increasing order of their Atomic number.
Mendeleev predicted the existence of which element/elements in the periodic table?- a)Gallium
- b)Sodium and germanium
- c)Gallium and germanium
- d)Germanium and Gold
Correct answer is option 'C'. Can you explain this answer?
Mendeleev predicted the existence of which element/elements in the periodic table?
a)
Gallium
b)
Sodium and germanium
c)
Gallium and germanium
d)
Germanium and Gold
|
|
Neha Joshi answered |
Gallium and Germanium were the elements not discovered at that time and Mendeleev put gaps in the periodic table.
Gallium was called as Eka aluminium
Germanium was called as Eka silicon
Gallium was called as Eka aluminium
Germanium was called as Eka silicon
How many orbitals are filled in second period?- a)6
- b)3
- c)1
- d)4
Correct answer is option 'D'. Can you explain this answer?
How many orbitals are filled in second period?
a)
6
b)
3
c)
1
d)
4
|
|
Lavanya Menon answered |
The 4 orbitals filled in second period are one 2s (with 2 electrons) and three 2p (with 6 electrons).
Newland arranged elements in increasing order of atomic weights and noted that every eighth element had properties similar to:- a)Third element
- b)Fourth element
- c)Second element
- d)First element
Correct answer is option 'D'. Can you explain this answer?
Newland arranged elements in increasing order of atomic weights and noted that every eighth element had properties similar to:
a)
Third element
b)
Fourth element
c)
Second element
d)
First element
|
|
Hansa Sharma answered |
According to Newlands' law of octaves when the elements are arranged in order of increasing atomic weights then every eighth element has properties similar to that of the first element.
14 elements after actinium is called
a) Lanthanides
b) Actinides
c) D-block elements
d) P block elements
Correct answer is option 'B'. Can you explain this answer?
|
|
Neha Sharma answered |
The correct answer is Option B.
14 elements after actinium is called Actinides.
The largest atomic radius among the following is of:- a)Na
- b)Mg
- c)K
- d)Ca
Correct answer is option 'C'. Can you explain this answer?
The largest atomic radius among the following is of:
a)
Na
b)
Mg
c)
K
d)
Ca
|
|
Geetika Shah answered |
Atomic radius decreases along period, the very next elements K ,Ca has K has higher atomic radius.
What is the principal quantum number for second period?a)1b)3c)2d)4Correct answer is option 'C'. Can you explain this answer?
|
|
Krishna Iyer answered |
Principle quantum no is defined as the no of shells an element possesses. Since every element in the 2nd period has 2 shells, therefore, principle quantum no for 2nd period is 2.
The maximum number of elements present in seventh period of the modern periodic table is:- a)32
- b)8
- c)2
- d)18
Correct answer is option 'A'. Can you explain this answer?
The maximum number of elements present in seventh period of the modern periodic table is:
a)
32
b)
8
c)
2
d)
18
|
|
Anjali Iyer answered |
The total number of electrons that can be accommodated in seventh period are 2 ( in 7s) + 14(in 5f) + 10(in 6d )+ 6(in 7p) = 32. The maximum number of elements present in it is 32.
What is the maximum number of electrons that can be filled in 3d subshell?- a)2
- b)14
- c)6
- d)10
Correct answer is option 'D'. Can you explain this answer?
What is the maximum number of electrons that can be filled in 3d subshell?
a)
2
b)
14
c)
6
d)
10
|
|
Shreya Gupta answered |
There are 5 orbitals in d subshell and each orbital can accommodate 2 electrons. Hence maximum number of electrons that can be filled in 3d subshell is 10.
For alkali metals, which one of the following trends is INCORRECT?
- a)Hydration energy: Li > Na > K > Rb
- b)Ionization energy: Li > Na > K > Rb
- c)Atomic size: Li < Na < K < Rb
- d)Density: Li < Na < K < Rb
Correct answer is option 'D'. Can you explain this answer?
For alkali metals, which one of the following trends is INCORRECT?
a)
Hydration energy: Li > Na > K > Rb
b)
Ionization energy: Li > Na > K > Rb
c)
Atomic size: Li < Na < K < Rb
d)
Density: Li < Na < K < Rb
|
|
Om Desai answered |
Density: Li < Na < K < Rb.
The density of K is lower than that of Na. Thus, option D is incorrect. The correct trend is
Li < K < Na < Rb
The density of K is lower than that of Na. Thus, option D is incorrect. The correct trend is
Li < K < Na < Rb
Which of the following elements has the largest ionisation enthalpy?- a)O
- b)F
- c)C
- d)N
Correct answer is option 'B'. Can you explain this answer?
Which of the following elements has the largest ionisation enthalpy?
a)
O
b)
F
c)
C
d)
N
|
|
Gaurav Kumar answered |
Ionisation energy increases along a period. The very next elements C,N,O,F results F with higher ionisation energy.
Among the following the element with highest first ionization energy is:- a)C
- b)Al
- c)Be
- d)Si
Correct answer is option 'A'. Can you explain this answer?
Among the following the element with highest first ionization energy is:
a)
C
b)
Al
c)
Be
d)
Si
|
|
Mira Sharma answered |
Carbon has the highest first ionisation energy because ionisation energy decreases down the group and increases from left to right in a period.
For noble gases radius is measured as:- a)Atomic radius
- b)Ionic radius
- c)van der Waals radius
- d)Metallic radius
Correct answer is option 'C'. Can you explain this answer?
For noble gases radius is measured as:
a)
Atomic radius
b)
Ionic radius
c)
van der Waals radius
d)
Metallic radius
|
|
Neha Joshi answered |
It is believed that Noble gases don't form (or rather don't easily form bonds). This way only Van der Waals radius is measured.
The rationale here is based on the fact that noble gases have very low chemical reactivity and their atomic radii are non bonded.
Electron gain enthalpy is most negative for:- a)Fluorine
- b)Sulphur
- c)Phosphorus
- d)Chlorine
Correct answer is option 'D'. Can you explain this answer?
Electron gain enthalpy is most negative for:
a)
Fluorine
b)
Sulphur
c)
Phosphorus
d)
Chlorine
|
|
Pooja Shah answered |
The negative electron gain enthalpy of fluorine is less than that of chlorine. It is due to small size of fluorine atom. As a result, there are strong interelectronic repulsions in the relatively small 2p orbitals of fluorine and thus, the incoming electron does not experience much attraction.
What happens to atomic radius on going from left to right in a period in a periodic table?- a)Remains constant
- b)Increases
- c)Decreases
- d)Decreases first and then remains constant
Correct answer is option 'C'. Can you explain this answer?
What happens to atomic radius on going from left to right in a period in a periodic table?
a)
Remains constant
b)
Increases
c)
Decreases
d)
Decreases first and then remains constant
|
|
Preeti Iyer answered |
Atomic radius is generally decreases from left to right along a period because the electrons are added to same shell due to this they experience a great force from the nucleus. While atomic radiusincreases in group from top to bottom cause new shells are added and the electrons are bring away from the nucleus.
Which of following statement is incorrect regarding ionization enthalpy:- a)The greater increase in ionization enthalpy is experienced on removal of electron from core noble gas configuration
- b)Ionization enthalpy increases on moving from left to right along a period
- c)Ionization enthalpy decreases down the group
- d)Removal of electrons from orbitals bearing lower n value is easier than from orbital having higher n value
Correct answer is option 'D'. Can you explain this answer?
Which of following statement is incorrect regarding ionization enthalpy:
a)
The greater increase in ionization enthalpy is experienced on removal of electron from core noble gas configuration
b)
Ionization enthalpy increases on moving from left to right along a period
c)
Ionization enthalpy decreases down the group
d)
Removal of electrons from orbitals bearing lower n value is easier than from orbital having higher n value
|
|
Pooja Shah answered |
The option (d) represents incorrect statement. It states that
"Removal of electron from orbitals bearing lower n value is easier than from orbital having higher n value".
The correct statement is "Removal of electron from orbitals bearing lower n value is difficult than from orbital having higher n value".
The attraction between the nucleus and the electron present in orbital having lower n value is higher than the attraction between the nucleus and the electron present in orbital having higher n value.
"Removal of electron from orbitals bearing lower n value is easier than from orbital having higher n value".
The correct statement is "Removal of electron from orbitals bearing lower n value is difficult than from orbital having higher n value".
The attraction between the nucleus and the electron present in orbital having lower n value is higher than the attraction between the nucleus and the electron present in orbital having higher n value.
The 3d transition series starts from which atomic number?- a)19
- b)21
- c)20
- d)22
Correct answer is option 'B'. Can you explain this answer?
The 3d transition series starts from which atomic number?
a)
19
b)
21
c)
20
d)
22
|
|
Ishani Pillai answered |
The 3d transition series starts from atomic number 21, which is the element Scandium (Sc).
Explanation:
- The transition elements or transition metals are a group of elements in the periodic table that have partially filled d orbitals in their valence shells.
- The 3d transition series refers to the elements that have their d orbitals filled progressively, from scandium (Sc) to zinc (Zn), as the atomic number increases.
- Scandium has an atomic number of 21, which means it has 21 protons in its nucleus and 21 electrons surrounding it.
- Scandium is the first element in the 3d transition series and has one electron in its 3d orbital.
- The other elements in the 3d transition series are titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn).
- These elements have their d orbitals filled progressively, from 1 electron in Sc to 10 electrons in Zn.
- The 4d and 5d transition series follow the same pattern, with the d orbitals being progressively filled from the first element in each series.
Therefore, the correct answer is option B, 21.
Explanation:
- The transition elements or transition metals are a group of elements in the periodic table that have partially filled d orbitals in their valence shells.
- The 3d transition series refers to the elements that have their d orbitals filled progressively, from scandium (Sc) to zinc (Zn), as the atomic number increases.
- Scandium has an atomic number of 21, which means it has 21 protons in its nucleus and 21 electrons surrounding it.
- Scandium is the first element in the 3d transition series and has one electron in its 3d orbital.
- The other elements in the 3d transition series are titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn).
- These elements have their d orbitals filled progressively, from 1 electron in Sc to 10 electrons in Zn.
- The 4d and 5d transition series follow the same pattern, with the d orbitals being progressively filled from the first element in each series.
Therefore, the correct answer is option B, 21.
The last element of actinoid series is:- a)Lutetium
- b)Thorium
- c)Cerium
- d)Lawrencium
Correct answer is option 'D'. Can you explain this answer?
The last element of actinoid series is:
a)
Lutetium
b)
Thorium
c)
Cerium
d)
Lawrencium
|
|
Pooja Mehta answered |
The last element in the actinoid series is lawrencium, Lr. Its atomic number is 103 and its electronic configuration is[Rn]5 f146d17s2. The most common oxidation state displayed by it is +3; because after losing 3 electrons it attains stable f14 configuration.
For IUPAC nomenclature of elements with atomic number greater than 100, the abbreviation used for digit zero is:- a)o
- b)MN
- c)n
- d)N
Correct answer is 'C'. Can you explain this answer?
For IUPAC nomenclature of elements with atomic number greater than 100, the abbreviation used for digit zero is:
a)
o
b)
MN
c)
n
d)
N
|
|
Mira Sharma answered |
For IUPAC nomenclature of elements with atomic number greater than 100, the abbreviation used for digit zero is n and name is nil.
The distribution of electrons into orbitals of an atom is called its:- a)Electronic configuration
- b)Atomic configuration
- c)Molecular configuration
- d)Configuration
Correct answer is option 'A'. Can you explain this answer?
The distribution of electrons into orbitals of an atom is called its:
a)
Electronic configuration
b)
Atomic configuration
c)
Molecular configuration
d)
Configuration

|
Vaishnavi Bajaj answered |
The arrangement of electrons in different shells and sub-shells is known as the electronic configuration of a particular element. The electronic configuration diagram represents an element in its ground state or stable state. There are a set of rules to remember while distribution off electrons in different orbits.
Electron gain enthalpy values of noble gases are positive because:- a)They are all gases
- b)They are monoatomic
- c)They have smallest size
- d)They have stable electronic configuration
Correct answer is option 'D'. Can you explain this answer?
Electron gain enthalpy values of noble gases are positive because:
a)
They are all gases
b)
They are monoatomic
c)
They have smallest size
d)
They have stable electronic configuration
|
|
Preeti Iyer answered |
Electron gain enthalpy values of noble gases are positive because they have stable electronic configuration and thus have no tendency to take additional electron.
Which of the following will have the most negative electron gain enthalpy and which one the least negative? P, S, Cl, F.- a)Cl, P
- b)P,Cl
- c)P,S
- d)none
Correct answer is option 'A'. Can you explain this answer?
Which of the following will have the most negative electron gain enthalpy and which one the least negative? P, S, Cl, F.
a)
Cl, P
b)
P,Cl
c)
P,S
d)
none
|
|
Krishna Iyer answered |
Cl just need one more electrons to get fully filled electronic configuration so it has most negative electron gain enthalpy while P has d3 configuration which is already stable so it cannot accept one electron extra easily so have large positive electron gain enthalpy.
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of + 2 oxidation state will be there in which of the following order? (At. nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)- a)Fe > Mn > Co > Cr
- b)Mn > Fe > Cr > Co
- c)Cr > Mn > Co > Fe
- d)Co > Mn > Fe > Cr
Correct answer is option 'B'. Can you explain this answer?
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of + 2 oxidation state will be there in which of the following order? (At. nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
a)
Fe > Mn > Co > Cr
b)
Mn > Fe > Cr > Co
c)
Cr > Mn > Co > Fe
d)
Co > Mn > Fe > Cr

|
Gaurav Saini answered |
Mn has most stable +2 stable because Mn+2 had d5 configuration which is most stable.
Which of the following elements will have highest ionisation energy?- a)1s2 2s2 2p6 3s1
- b)1s2 2s2 2p6 3s2 3p3
- c)1s2 2s2 2p6 3s2 3p4
- d)1s2 2s2 2p6 3s2 3p1
Correct answer is option 'B'. Can you explain this answer?
Which of the following elements will have highest ionisation energy?
a)
1s2 2s2 2p6 3s1
b)
1s2 2s2 2p6 3s2 3p3
c)
1s2 2s2 2p6 3s2 3p4
d)
1s2 2s2 2p6 3s2 3p1
|
|
Jaspreet answered |
In half filled and fully filled orbitals it's difficult to remove an electron from its outermost shell cause it's Highly stable electronic configuration so it's option B !!!
Mendeleev's Periodic Table was arranged primarily based on which property of elements?- a)Atomic mass
- b)Electronegativity
- c)Ionization energy
- d)Atomic radius
Correct answer is option 'A'. Can you explain this answer?
Mendeleev's Periodic Table was arranged primarily based on which property of elements?
a)
Atomic mass
b)
Electronegativity
c)
Ionization energy
d)
Atomic radius

|
Lead Academy answered |
Mendeleev's Periodic Table was initially arranged based on increasing atomic mass. He noticed that elements with similar properties appeared at regular intervals when arranged in order of increasing atomic mass.
Chapter doubts & questions for Classification of Elements & Periodicity in Properties - Chemistry Class 11 2025 is part of Class 11 exam preparation. The chapters have been prepared according to the Class 11 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Class 11 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Classification of Elements & Periodicity in Properties - Chemistry Class 11 in English & Hindi are available as part of Class 11 exam.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Chemistry Class 11
170 videos|269 docs|130 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup