All Exams >
Civil Engineering (CE) >
Environmental Engineering >
All Questions
All questions of Quality Parameters of Water for Civil Engineering (CE) Exam
One True Colour Unit (TCU) is the colour produced by
- a)one mg of formazin in one litre of distilled water
- b)one mg of silicon in one litre of distilled water
- c)one mg of ferric silicon in one litre of distilled water
- d)one mg of platinum as chloroplatinate ions in one litre of distilled water
Correct answer is option 'D'. Can you explain this answer?
One True Colour Unit (TCU) is the colour produced by
a)
one mg of formazin in one litre of distilled water
b)
one mg of silicon in one litre of distilled water
c)
one mg of ferric silicon in one litre of distilled water
d)
one mg of platinum as chloroplatinate ions in one litre of distilled water

|
Telecom Tuners answered |
The correct option is D 1 mg/L platinum in form of chloroplatinate ion
TCU is the colour unit and represented by colour produced by 1 mg/L of platinum in the form of chloroplatinate ion dissolved in 1 L of distilled water.
TCU is the colour unit and represented by colour produced by 1 mg/L of platinum in the form of chloroplatinate ion dissolved in 1 L of distilled water.
The maximum permissible limit for fluoride in drinking water is- a)0.1 mg//
- b)1.5 mg/I
- c)5 mg/l
- d)10 mg/l
Correct answer is option 'B'. Can you explain this answer?
The maximum permissible limit for fluoride in drinking water is
a)
0.1 mg//
b)
1.5 mg/I
c)
5 mg/l
d)
10 mg/l

|
Rithika Kaur answered |
A fluoride concentration of less than 0.8-1.0 ppm may be harmful and may cause dental caries (tooth decay) due to the formation of excessive cavities in the teeth of young children during calcination of their permanent teeth. Higher fluoride concentrations, greater than 1.5 ppm or so, may again be harmful, causing spotting and discolouration of teeth, (a disease called fluorosis), which with continued excessive consumption of fuorides, may even cause deformation of bones.
What is the Kjeldahl nitrogen in sewage?- a)Sum of ammonia nitrogen and nitrites.
- b)Sum of organic nitrogen and nitrates.
- c)Sum of ammonia nitrogen and organic nitrogen.
- d)Sum of nitrites and nitrates.
Correct answer is option 'C'. Can you explain this answer?
What is the Kjeldahl nitrogen in sewage?
a)
Sum of ammonia nitrogen and nitrites.
b)
Sum of organic nitrogen and nitrates.
c)
Sum of ammonia nitrogen and organic nitrogen.
d)
Sum of nitrites and nitrates.
|
|
Tanvi Shah answered |
Nitrogen in water:
The nitrogen in water indicates organic contamination of water i.e when water is contaminated by sewage then nitrogen compounds are traced in water. We can trace the following compounds,
The nitrogen in water indicates organic contamination of water i.e when water is contaminated by sewage then nitrogen compounds are traced in water. We can trace the following compounds,
- Ammonia nitrogen
- Organic nitrogen or Albuminoid nitrogen
- Nitrites
- Nitrates
Nitrogen compounds are measured by colorimetry
Total Kjeldahl nitrogen (TKN):
It is the sum of organic nitrogen, ammonia (NH3), and ammonium (NH4+) in the chemical analysis of soil, water, and wastewater.
To calculate Total Nitrogen (TN), the concentrations of nitrate-N and nitrite-N are determined and added to the total Kjeldahl nitrogen
Kjehldahl Nitrogen = Organic nitrogen + free ammonia
Total Kjeldahl nitrogen (TKN):
It is the sum of organic nitrogen, ammonia (NH3), and ammonium (NH4+) in the chemical analysis of soil, water, and wastewater.
To calculate Total Nitrogen (TN), the concentrations of nitrate-N and nitrite-N are determined and added to the total Kjeldahl nitrogen
Kjehldahl Nitrogen = Organic nitrogen + free ammonia
Which of the following method is used to forecast the population of old and very large city?- a)Arithmetical increase method
- b)Geometric progression method
- c)Graphical method
- d)Logistic curve method
Correct answer is option 'A'. Can you explain this answer?
Which of the following method is used to forecast the population of old and very large city?
a)
Arithmetical increase method
b)
Geometric progression method
c)
Graphical method
d)
Logistic curve method

|
Kirti Sharma answered |
Arithmetical increase method
The arithmetical increase method is used to forecast the population of old and very large cities. This method assumes a constant increase in population over time, based on an arithmetic progression.
Steps in the arithmetical increase method:
1. Collect historical population data: Gather data on the population of the city over a period of time. The more data available, the more accurate the forecast will be.
2. Determine the time period: Decide on the time period for which the population forecast is required. This could be short-term or long-term, depending on the purpose of the forecast.
3. Calculate the arithmetic mean: Find the arithmetic mean of the population data collected. This is done by summing up all the population values and dividing it by the number of data points.
4. Compute the arithmetic difference: Calculate the arithmetic difference between consecutive population values. This is done by subtracting the population of a previous year from the population of the next year.
5. Find the average difference: Calculate the average difference by summing up all the arithmetic differences and dividing it by the number of differences.
6. Forecast the population: Use the average difference to forecast the population for the desired time period. Multiply the average difference by the number of years in the forecast period and add it to the latest population value.
7. Verify the forecast: Compare the forecasted population with the actual population values for the corresponding years. Adjustments may be required based on any significant changes or factors not considered in the arithmetical increase method.
Advantages of the arithmetical increase method:
- Simple and easy to use.
- Does not require complex calculations or assumptions.
- Provides a rough estimate of population growth.
Limitations of the arithmetical increase method:
- Assumes a constant rate of population growth, which may not hold true in reality.
- Does not account for factors such as migration, birth rates, or government policies that can affect population growth.
- Accuracy decreases over longer forecast periods.
- Relies heavily on historical data, which may not accurately represent future trends.
The arithmetical increase method is used to forecast the population of old and very large cities. This method assumes a constant increase in population over time, based on an arithmetic progression.
Steps in the arithmetical increase method:
1. Collect historical population data: Gather data on the population of the city over a period of time. The more data available, the more accurate the forecast will be.
2. Determine the time period: Decide on the time period for which the population forecast is required. This could be short-term or long-term, depending on the purpose of the forecast.
3. Calculate the arithmetic mean: Find the arithmetic mean of the population data collected. This is done by summing up all the population values and dividing it by the number of data points.
4. Compute the arithmetic difference: Calculate the arithmetic difference between consecutive population values. This is done by subtracting the population of a previous year from the population of the next year.
5. Find the average difference: Calculate the average difference by summing up all the arithmetic differences and dividing it by the number of differences.
6. Forecast the population: Use the average difference to forecast the population for the desired time period. Multiply the average difference by the number of years in the forecast period and add it to the latest population value.
7. Verify the forecast: Compare the forecasted population with the actual population values for the corresponding years. Adjustments may be required based on any significant changes or factors not considered in the arithmetical increase method.
Advantages of the arithmetical increase method:
- Simple and easy to use.
- Does not require complex calculations or assumptions.
- Provides a rough estimate of population growth.
Limitations of the arithmetical increase method:
- Assumes a constant rate of population growth, which may not hold true in reality.
- Does not account for factors such as migration, birth rates, or government policies that can affect population growth.
- Accuracy decreases over longer forecast periods.
- Relies heavily on historical data, which may not accurately represent future trends.
The standard turbidity produced by one mg of silicon dioxide (silica) in one litre of distilled water, is called- a)one Jackson turbidity unit (JTU)
- b)one Formazin turbidity unit (FTU)
- c)one Nephelometry turbidity unit (NTU)
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
The standard turbidity produced by one mg of silicon dioxide (silica) in one litre of distilled water, is called
a)
one Jackson turbidity unit (JTU)
b)
one Formazin turbidity unit (FTU)
c)
one Nephelometry turbidity unit (NTU)
d)
None of the above
|
|
Rajeev Menon answered |
The standard turbidity produced by one mg of silicon dioxide (silica) in one litre of distilled water is called one Formazin turbidity unit (FTU). Formazin is a synthetic compound that is used as a standard reference material for turbidity measurements.
Turbidity is a measure of the cloudiness or clarity of a liquid. It is commonly used to measure the clarity of water and is often used as an indicator of water quality. Turbidity is typically measured in units of Formazin turbidity units (FTU), Nephelometry turbidity units (NTU), or Jackson turbidity units (JTU).
In general, the lower the turbidity of a water sample, the clearer and more transparent it is. Water with a high turbidity may appear cloudy or murky, and may contain suspended particles or other contaminants.
Which one of the following organisms is responsible for enteric fever?- a)ECHO
- b)Salmonella typhi
- c)Entamoeba histolytica
- d)Echinococcus
Correct answer is option 'B'. Can you explain this answer?
Which one of the following organisms is responsible for enteric fever?
a)
ECHO
b)
Salmonella typhi
c)
Entamoeba histolytica
d)
Echinococcus

|
Jyoti Choudhury answered |
Salmonella typhi and Salmonella paratyphi (bacteria) cause enteric fever.
Enteric Cytopathogenic Human Orphan ECHO (virus) cause aseptic meningitis, epidemic exanthem, infantile diarrhoea.
Entamoeba histolytica (protozoa) cause dimebiasis (amebic dysentry, amebic enteritis, amebic colitis). Echmococcus (helminth) cause enchinococcosis (hydlatidosis, granulosis, dog tapeworm).
Enteric Cytopathogenic Human Orphan ECHO (virus) cause aseptic meningitis, epidemic exanthem, infantile diarrhoea.
Entamoeba histolytica (protozoa) cause dimebiasis (amebic dysentry, amebic enteritis, amebic colitis). Echmococcus (helminth) cause enchinococcosis (hydlatidosis, granulosis, dog tapeworm).
The spacing between two bars in medium size screen ranges from- a)20 – 50 mm
- b)20 – 40 mm
- c)10 – 20 mm
- d)10 – 30 mm
Correct answer is option 'B'. Can you explain this answer?
The spacing between two bars in medium size screen ranges from
a)
20 – 50 mm
b)
20 – 40 mm
c)
10 – 20 mm
d)
10 – 30 mm
|
|
Lalit Yadav answered |
Some important points about screens in the screening process
- The coarse screen consists of parallel iron rods are placed vertically or at a slight slope at about 25 to 50 mm apart
- The fine screen is usually made of woven wire mesh with an opening not more than 6 mm square
- The spacing between two bars in medium size screen ranges from 15 to 40 mm
- The clear spacing between the bars may be in the range of 15 mm to 75 mm in case of a mechanically cleaned bar screen
- However, for the manually cleaned bar screen, the clear spacing used is in the range 25 mm to 50 mm
Electrical conductivity (EC) of water and total dissolved solids (TDS) are interrelated. The value of EC will- a)decreases with increase in TDS
- b)increases with increase in TDS
- c)decreases initially and then increase with increase in TDS
- d)increases initially and then decrease with increase in TDS
Correct answer is option 'B'. Can you explain this answer?
Electrical conductivity (EC) of water and total dissolved solids (TDS) are interrelated. The value of EC will
a)
decreases with increase in TDS
b)
increases with increase in TDS
c)
decreases initially and then increase with increase in TDS
d)
increases initially and then decrease with increase in TDS

|
Bijoy Chauhan answered |
Introduction:
Electrical conductivity (EC) is a measure of a solution's ability to conduct an electric current. It is influenced by the presence of charged ions in the solution. Total Dissolved Solids (TDS) refers to the total amount of inorganic and organic substances dissolved in water, including salts, minerals, metals, and other compounds.
Interrelation between EC and TDS:
EC and TDS are closely related due to the presence of dissolved ions in water. As the concentration of dissolved ions increases, both EC and TDS values also increase. This is because the ions present in the water act as charge carriers and facilitate the flow of electric current.
Explanation of the correct answer (Option B):
The EC value of water increases with an increase in TDS concentration. This means that as the amount of dissolved solids in water increases, the ability of the water to conduct electric current also increases. Therefore, option B is the correct answer.
Reason for the increase in EC with an increase in TDS:
When dissolved solids, such as salts, minerals, and other compounds, are present in water, they dissociate into charged ions. These ions have the ability to carry electric current. As the concentration of dissolved solids increases, the number of charged ions in the water also increases. This results in an increase in the conductivity of water, as more ions are available to carry the electric current.
Example:
For example, consider two water samples: Sample A with a low TDS concentration and Sample B with a high TDS concentration. When an electric current is passed through both samples, Sample B will have a higher EC value compared to Sample A. This is because Sample B contains a higher concentration of dissolved solids, resulting in a greater number of charged ions available to conduct the electric current.
Conclusion:
In summary, the EC of water is directly related to the TDS concentration. As the amount of dissolved solids increases, the ability of water to conduct electric current also increases. Therefore, the correct answer is option B, which states that the EC increases with an increase in TDS concentration.
Electrical conductivity (EC) is a measure of a solution's ability to conduct an electric current. It is influenced by the presence of charged ions in the solution. Total Dissolved Solids (TDS) refers to the total amount of inorganic and organic substances dissolved in water, including salts, minerals, metals, and other compounds.
Interrelation between EC and TDS:
EC and TDS are closely related due to the presence of dissolved ions in water. As the concentration of dissolved ions increases, both EC and TDS values also increase. This is because the ions present in the water act as charge carriers and facilitate the flow of electric current.
Explanation of the correct answer (Option B):
The EC value of water increases with an increase in TDS concentration. This means that as the amount of dissolved solids in water increases, the ability of the water to conduct electric current also increases. Therefore, option B is the correct answer.
Reason for the increase in EC with an increase in TDS:
When dissolved solids, such as salts, minerals, and other compounds, are present in water, they dissociate into charged ions. These ions have the ability to carry electric current. As the concentration of dissolved solids increases, the number of charged ions in the water also increases. This results in an increase in the conductivity of water, as more ions are available to carry the electric current.
Example:
For example, consider two water samples: Sample A with a low TDS concentration and Sample B with a high TDS concentration. When an electric current is passed through both samples, Sample B will have a higher EC value compared to Sample A. This is because Sample B contains a higher concentration of dissolved solids, resulting in a greater number of charged ions available to conduct the electric current.
Conclusion:
In summary, the EC of water is directly related to the TDS concentration. As the amount of dissolved solids increases, the ability of water to conduct electric current also increases. Therefore, the correct answer is option B, which states that the EC increases with an increase in TDS concentration.
The permissible limit of nitrate content in potable water is:- a)25 ppm
- b)100 ppm
- c)10 ppm
- d)45 ppm
Correct answer is option 'D'. Can you explain this answer?
The permissible limit of nitrate content in potable water is:
a)
25 ppm
b)
100 ppm
c)
10 ppm
d)
45 ppm
|
|
Lalit Yadav answered |
The permissible limits of various compounds are as follows:
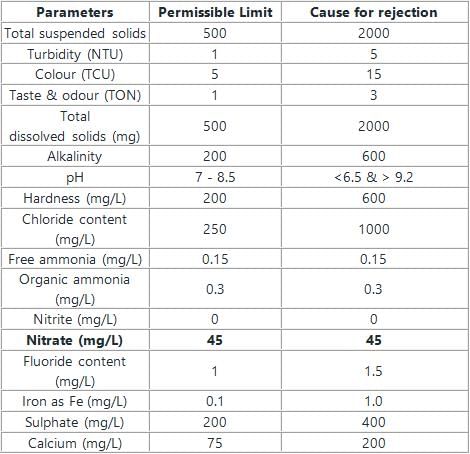

The population of a city in the first three continuous years is given as 6000, 8000 and 10000 respectively. What is the population of the city in the fourth continuous year, according to the geometric increase method?- a)11500
- b)12000
- c)12870
- d)14000
Correct answer is option 'C'. Can you explain this answer?
The population of a city in the first three continuous years is given as 6000, 8000 and 10000 respectively. What is the population of the city in the fourth continuous year, according to the geometric increase method?
a)
11500
b)
12000
c)
12870
d)
14000

|
Hiral Sharma answered |
Given information:
Population in the first year = 6000
Population in the second year = 8000
Population in the third year = 10000
Geometric Increase Method:
In the geometric increase method, we use the formula:
Pn = P0 * (1 + r)^n
where
Pn = population in the nth year
P0 = initial population
r = rate of increase
n = number of years
To find the rate of increase, we can use the formula:
r = (P2 - P1) / P1
where
P1 = population in the first year
P2 = population in the second year
r = (8000 - 6000) / 6000
r = 0.3333
Using the above values in the formula for the fourth year, we get:
P4 = 6000 * (1 + 0.3333)^4
P4 = 12870
Therefore, the population of the city in the fourth continuous year, according to the geometric increase method, is 12870. Hence, the correct answer is option C.
Population in the first year = 6000
Population in the second year = 8000
Population in the third year = 10000
Geometric Increase Method:
In the geometric increase method, we use the formula:
Pn = P0 * (1 + r)^n
where
Pn = population in the nth year
P0 = initial population
r = rate of increase
n = number of years
To find the rate of increase, we can use the formula:
r = (P2 - P1) / P1
where
P1 = population in the first year
P2 = population in the second year
r = (8000 - 6000) / 6000
r = 0.3333
Using the above values in the formula for the fourth year, we get:
P4 = 6000 * (1 + 0.3333)^4
P4 = 12870
Therefore, the population of the city in the fourth continuous year, according to the geometric increase method, is 12870. Hence, the correct answer is option C.
The tolerance limit of hardness in water as per IS drinking water standard is specified as- a)200 mg/L
- b)750 mg/L
- c)600 mg/L
- d)500 mg/L
Correct answer is option 'C'. Can you explain this answer?
The tolerance limit of hardness in water as per IS drinking water standard is specified as
a)
200 mg/L
b)
750 mg/L
c)
600 mg/L
d)
500 mg/L

|
Bibek Mehra answered |
Explanation:
Hardness in Water:
Hardness in water refers to the concentration of dissolved minerals, primarily calcium and magnesium ions. These minerals are naturally present in water sources and can cause various issues, such as scale formation in pipes and appliances, decreased soap efficiency, and unpleasant taste.
IS Drinking Water Standard:
The Indian Standard (IS) for drinking water quality is specified by the Bureau of Indian Standards (BIS). This standard sets limits for various parameters, including hardness, to ensure the safety and quality of drinking water.
Limit of Hardness in Water:
According to the IS drinking water standard, the tolerance limit for hardness is specified as 600 mg/L.
Explanation of Options:
a) 200 mg/L: This option is incorrect as it is lower than the specified limit of 600 mg/L.
b) 750 mg/L: This option is incorrect as it is higher than the specified limit of 600 mg/L.
c) 600 mg/L: This option is correct as it matches the specified tolerance limit for hardness in water.
d) 500 mg/L: This option is incorrect as it is lower than the specified limit of 600 mg/L.
Importance of Specified Limit:
The specified limit of hardness in water is important to ensure that the water is safe for consumption and does not cause any adverse health effects. Excessive hardness can lead to the deposition of scale in water pipes, which can reduce the flow rate and increase maintenance costs. It can also affect the efficiency of water heaters, boilers, and other appliances that use water. By adhering to the specified limit, the quality and usability of drinking water can be maintained.
In conclusion, the correct answer is option 'C', which states that the tolerance limit of hardness in water as per the IS drinking water standard is specified as 600 mg/L.
Hardness in Water:
Hardness in water refers to the concentration of dissolved minerals, primarily calcium and magnesium ions. These minerals are naturally present in water sources and can cause various issues, such as scale formation in pipes and appliances, decreased soap efficiency, and unpleasant taste.
IS Drinking Water Standard:
The Indian Standard (IS) for drinking water quality is specified by the Bureau of Indian Standards (BIS). This standard sets limits for various parameters, including hardness, to ensure the safety and quality of drinking water.
Limit of Hardness in Water:
According to the IS drinking water standard, the tolerance limit for hardness is specified as 600 mg/L.
Explanation of Options:
a) 200 mg/L: This option is incorrect as it is lower than the specified limit of 600 mg/L.
b) 750 mg/L: This option is incorrect as it is higher than the specified limit of 600 mg/L.
c) 600 mg/L: This option is correct as it matches the specified tolerance limit for hardness in water.
d) 500 mg/L: This option is incorrect as it is lower than the specified limit of 600 mg/L.
Importance of Specified Limit:
The specified limit of hardness in water is important to ensure that the water is safe for consumption and does not cause any adverse health effects. Excessive hardness can lead to the deposition of scale in water pipes, which can reduce the flow rate and increase maintenance costs. It can also affect the efficiency of water heaters, boilers, and other appliances that use water. By adhering to the specified limit, the quality and usability of drinking water can be maintained.
In conclusion, the correct answer is option 'C', which states that the tolerance limit of hardness in water as per the IS drinking water standard is specified as 600 mg/L.
In which one of the following tests is the organic matter in the waste water used as food by microorganisms?- a)BOD
- b)Most probable number
- c)COD
- d)Chlorine demand
Correct answer is option 'A'. Can you explain this answer?
In which one of the following tests is the organic matter in the waste water used as food by microorganisms?
a)
BOD
b)
Most probable number
c)
COD
d)
Chlorine demand
|
|
Aditya Deshmukh answered |
The test in which the organic matter in the wastewater is used as food by microorganisms is the Biochemical Oxygen Demand (BOD) test.
The BOD test measures the amount of oxygen required by microorganisms to break down the organic matter in the water. The test involves measuring the dissolved oxygen in a sample of water at the start of the test (initial DO) and then again at the end of a 5-day incubation period. The difference between the two values is known as the BOD5 and it is used to indicate the degree of organic pollution present in the water. A higher BOD5 value indicates more organic matter present in water and thus more oxygen consumed.
COD test (Chemical Oxygen Demand) test also measures oxygen demand of the water, but it measures the amount of oxygen required by all oxidizing agents, both naturally occurring and those added, not just by the microorganisms.
The Most Probable Number (MPN) test, is a microbiological method used to estimate the number of viable microorganisms present in a sample of water.
Chlorine Demand is not a test which has any relation with organic matter, it measures the amount of chlorine required to neutralize all the oxidizable matter present in the water, it also helps to determine the chlorine dosage to be used for disinfection.
The odour of the water or the waste-water can be measured by a term called:- a)Turbidity
- b)Threshold Odour Number
- c)B.O.D
- d)C.O.D
Correct answer is option 'B'. Can you explain this answer?
The odour of the water or the waste-water can be measured by a term called:
a)
Turbidity
b)
Threshold Odour Number
c)
B.O.D
d)
C.O.D
|
|
Lalit Yadav answered |
Odour and taste
It is one of the parameters which define the physical property of water
The odour in water is measured by a device known as osmoscope and expressed in terms of Threshold Odour Number (TON)
TON is a dilution ratio at which odour is just detectable
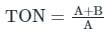
Where,
A = Volume of water sample tested in 'ml'
B = Volume of distilled water used in dilution in 'ml'
Capacity of osmoscope = A + B = 200 ml
There is no test for taste but senses of odour and taste are closely assosciated with each other. When water smell bad it also tastes bad.
The taste is expressed in terms of Flavoured Threshold Number (FTN)
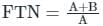
According to Drinking water standard TON < 3
It is one of the parameters which define the physical property of water
The odour in water is measured by a device known as osmoscope and expressed in terms of Threshold Odour Number (TON)
TON is a dilution ratio at which odour is just detectable
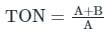
Where,
A = Volume of water sample tested in 'ml'
B = Volume of distilled water used in dilution in 'ml'
Capacity of osmoscope = A + B = 200 ml
There is no test for taste but senses of odour and taste are closely assosciated with each other. When water smell bad it also tastes bad.
The taste is expressed in terms of Flavoured Threshold Number (FTN)
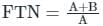
According to Drinking water standard TON < 3
Which of the following cations impart(s) pseudo hardness to water?- a)Calcium only
- b)Magnesium only
- c)Calcium and magnesium
- d)Sodium
Correct answer is option 'D'. Can you explain this answer?
Which of the following cations impart(s) pseudo hardness to water?
a)
Calcium only
b)
Magnesium only
c)
Calcium and magnesium
d)
Sodium

|
Ananya Sharma answered |
Monovalent cations impart pseudo-hardness. Hardness is the concentration of multivalent cations.
The maximum safe permissible limit of chlorides in domestic water supplies is- a)0.5 mg/l
- b)2.5 mg/l
- c)250 mg/l
- d)100 mg/l
Correct answer is option 'C'. Can you explain this answer?
The maximum safe permissible limit of chlorides in domestic water supplies is
a)
0.5 mg/l
b)
2.5 mg/l
c)
250 mg/l
d)
100 mg/l
|
|
Rajeev Menon answered |
The maximum safe permissible limit of chlorides in domestic water supplies varies depending on the guidelines and regulations in place in a particular region. In general, the maximum allowable concentration of chlorides in drinking water is set to protect against adverse effects on the taste and odor of the water, as well as to ensure the safety of the water for human consumption.
In the United States, the maximum contaminant level (MCL) for chlorides in drinking water is set at 250 mg/L by the Environmental Protection Agency (EPA). This MCL is based on the taste and odor of the water, and is intended to protect against water that tastes and smells too salty.
In the European Union, the maximum permissible limit for chlorides in drinking water is set at 200 mg/L by the European Union Council Directive 98/83/EC. This limit is based on the total dissolved solids (TDS) of the water, and is intended to protect against water that tastes and smells too salty or bitter.
In general, it is not correct to say that the maximum safe permissible limit of chlorides in domestic water supplies is 0.5 mg/L, 2.5 mg/L, or 100 mg/L. These values are lower than the generally accepted limits for chlorides in drinking water, and may not be sufficient to protect against adverse effects on the taste and odor of the water.
Match List-l (Equipment) with List-ll (Parameter) and select the correct answer using the codes given below the lists:
List-l
A. Tintometer
B. Nephelometer
C. Imhoffcone
D. Muffle furnaceList-ll
1. Temperature
2. Colour
3. Turbidity
4. Settleable solids
5. Volatile solidsCodes:
A B C D
(a) 4 3 1 5
(b) 2 5 4 3
(c) 4 5 1 3
(d) 2 3 4 5- a)a
- b)b
- c)c
- d)d
Correct answer is option 'D'. Can you explain this answer?
Match List-l (Equipment) with List-ll (Parameter) and select the correct answer using the codes given below the lists:
List-l
A. Tintometer
B. Nephelometer
C. Imhoffcone
D. Muffle furnace
List-l
A. Tintometer
B. Nephelometer
C. Imhoffcone
D. Muffle furnace
List-ll
1. Temperature
2. Colour
3. Turbidity
4. Settleable solids
5. Volatile solids
1. Temperature
2. Colour
3. Turbidity
4. Settleable solids
5. Volatile solids
Codes:
A B C D
(a) 4 3 1 5
(b) 2 5 4 3
(c) 4 5 1 3
(d) 2 3 4 5
A B C D
(a) 4 3 1 5
(b) 2 5 4 3
(c) 4 5 1 3
(d) 2 3 4 5
a)
a
b)
b
c)
c
d)
d

|
Shalini Deshpande answered |
Nephelometer is based on scattering principle for measurement of turbidity, Ituses formazin, a chemical compound, for standard. Formazin is more reproducible standard than SiO2. Tintometer is a color measuring instrument which compares colour of water in Nessler tubes which contain solutions of platinum Cobalt dissolved in water.
As per IS 10500:2012, the permissible limit of total dissolved solids (TDS), (in mg/l), in drinking water in the absence of an alternate source is:- a)1000
- b)500
- c)200
- d)2000
Correct answer is option 'D'. Can you explain this answer?
As per IS 10500:2012, the permissible limit of total dissolved solids (TDS), (in mg/l), in drinking water in the absence of an alternate source is:
a)
1000
b)
500
c)
200
d)
2000

|
Deepika Saha answered |
Permissible Limit of Total Dissolved Solids in Drinking Water
Permissible limit of total dissolved solids (TDS) in drinking water is defined by Indian Standards (IS) 10500:2012. TDS is the amount of inorganic and organic substances that are dissolved in water, including minerals, salts, and metals.
Permissible Limit
The permissible limit of TDS in drinking water in the absence of an alternate source is 2000 mg/l (milligrams per liter).
Explanation
The allowable limit of TDS in drinking water is established by the Bureau of Indian Standards (BIS) to guarantee that the water is safe to drink. Water with TDS concentrations exceeding the allowable limit may have a bitter or salty flavor, which is unpleasant to drink. It may also cause gastrointestinal problems if consumed over an extended period.
Conclusion
In conclusion, the permissible limit of TDS in drinking water in the absence of an alternate source is 2000 mg/l, as defined by Indian Standards (IS) 10500:2012. It is critical to adhere to these standards to guarantee the safety of drinking water.
Permissible limit of total dissolved solids (TDS) in drinking water is defined by Indian Standards (IS) 10500:2012. TDS is the amount of inorganic and organic substances that are dissolved in water, including minerals, salts, and metals.
Permissible Limit
The permissible limit of TDS in drinking water in the absence of an alternate source is 2000 mg/l (milligrams per liter).
Explanation
The allowable limit of TDS in drinking water is established by the Bureau of Indian Standards (BIS) to guarantee that the water is safe to drink. Water with TDS concentrations exceeding the allowable limit may have a bitter or salty flavor, which is unpleasant to drink. It may also cause gastrointestinal problems if consumed over an extended period.
Conclusion
In conclusion, the permissible limit of TDS in drinking water in the absence of an alternate source is 2000 mg/l, as defined by Indian Standards (IS) 10500:2012. It is critical to adhere to these standards to guarantee the safety of drinking water.
Water is considered ‘hard’, if its hardness is of the order of- a)50 ppm
- b)100ppm
- c)200 ppm
- d)300 ppm
Correct answer is option 'C'. Can you explain this answer?
Water is considered ‘hard’, if its hardness is of the order of
a)
50 ppm
b)
100ppm
c)
200 ppm
d)
300 ppm

|
Juhi Choudhary answered |
Hardness of Water
Hardness of water is a measure of the concentration of divalent cations, primarily calcium (Ca2+) and magnesium (Mg2+), in water. It is usually expressed in parts per million (ppm) of calcium carbonate (CaCO3).
Threshold for Hard Water
When the hardness of water is around 200 ppm or higher, it is considered to be hard water. This level of hardness can lead to various issues such as scale buildup in pipes and appliances, reduced effectiveness of soaps and detergents, and potentially negative impacts on skin and hair.
Options Analysis
a) 50 ppm - Water with 50 ppm hardness would be considered soft water, as it falls below the threshold for hard water.
b) 100 ppm - Water with 100 ppm hardness is still within the range of soft water and does not qualify as hard water.
c) 200 ppm - Water with 200 ppm hardness is at the threshold for hard water, making it the correct answer in this case.
d) 300 ppm - Water with 300 ppm hardness would also be considered hard water, as it exceeds the threshold level.
Conclusion
In summary, water is considered 'hard' when its hardness is around 200 ppm or higher. This level of hardness can have various implications for household use and may require appropriate treatment to mitigate its effects.
Hardness of water is a measure of the concentration of divalent cations, primarily calcium (Ca2+) and magnesium (Mg2+), in water. It is usually expressed in parts per million (ppm) of calcium carbonate (CaCO3).
Threshold for Hard Water
When the hardness of water is around 200 ppm or higher, it is considered to be hard water. This level of hardness can lead to various issues such as scale buildup in pipes and appliances, reduced effectiveness of soaps and detergents, and potentially negative impacts on skin and hair.
Options Analysis
a) 50 ppm - Water with 50 ppm hardness would be considered soft water, as it falls below the threshold for hard water.
b) 100 ppm - Water with 100 ppm hardness is still within the range of soft water and does not qualify as hard water.
c) 200 ppm - Water with 200 ppm hardness is at the threshold for hard water, making it the correct answer in this case.
d) 300 ppm - Water with 300 ppm hardness would also be considered hard water, as it exceeds the threshold level.
Conclusion
In summary, water is considered 'hard' when its hardness is around 200 ppm or higher. This level of hardness can have various implications for household use and may require appropriate treatment to mitigate its effects.
What is the acceptable limit for copper in water?- a)0.02 mg/l
- b)1.5 mg/l
- c)5 mg/l
- d)0.05 mg/l
Correct answer is option 'D'. Can you explain this answer?
What is the acceptable limit for copper in water?
a)
0.02 mg/l
b)
1.5 mg/l
c)
5 mg/l
d)
0.05 mg/l

|
Preethi Choudhury answered |
Acceptable Limit for Copper in Water
The acceptable limit for copper in water is 0.05 mg/l (milligrams per liter). This limit is set to ensure that the concentration of copper in drinking water remains within a safe range that does not pose significant health risks to humans. Exceeding this limit may result in potential adverse effects on human health and the environment.
Copper is a naturally occurring element that can be found in rocks, soil, water, and various products. It is an essential nutrient for humans and animals, but excessive exposure to copper can have detrimental effects on health. Therefore, regulatory authorities have established limits to control the concentration of copper in water sources.
Key Points:
- Acceptable limit for copper in water: 0.05 mg/l
- Limit is set to ensure human health and environmental safety
- Excessive copper exposure can have adverse effects
Health Effects of Excessive Copper Exposure
Excessive copper in drinking water can lead to various health effects, including acute and chronic toxicity. Some of the potential health effects of copper exposure include:
1. Gastrointestinal Issues: Consuming water with high copper levels can cause nausea, vomiting, abdominal pain, and diarrhea.
2. Liver and Kidney Damage: Prolonged exposure to high copper levels may lead to liver and kidney damage, impairing their normal functions.
3. Central Nervous System Effects: Copper accumulation in the brain can cause neurological symptoms such as headaches, dizziness, and irritability.
4. Hematological Effects: High copper levels can interfere with the production of red blood cells, leading to anemia or other blood disorders.
5. Carcinogenic Potential: Some studies suggest that long-term exposure to high copper concentrations may increase the risk of certain types of cancers.
Monitoring and Compliance
To ensure compliance with the acceptable limit for copper in water, regular monitoring and testing of water sources are necessary. Public water supplies are typically regulated by national or regional authorities that establish guidelines and standards for water quality, including copper concentration.
Water treatment processes, such as filtration and chemical treatment, can help reduce copper levels in drinking water. Additionally, proper maintenance of plumbing systems and pipes can minimize the leaching of copper into the water supply.
Conclusion
The acceptable limit for copper in water is 0.05 mg/l, which is set to protect human health and the environment. Exceeding this limit can result in various health effects, including gastrointestinal issues, liver and kidney damage, neurological symptoms, and hematological disorders. Regular monitoring and compliance with water quality standards are essential to ensure the safety of drinking water.
The acceptable limit for copper in water is 0.05 mg/l (milligrams per liter). This limit is set to ensure that the concentration of copper in drinking water remains within a safe range that does not pose significant health risks to humans. Exceeding this limit may result in potential adverse effects on human health and the environment.
Copper is a naturally occurring element that can be found in rocks, soil, water, and various products. It is an essential nutrient for humans and animals, but excessive exposure to copper can have detrimental effects on health. Therefore, regulatory authorities have established limits to control the concentration of copper in water sources.
Key Points:
- Acceptable limit for copper in water: 0.05 mg/l
- Limit is set to ensure human health and environmental safety
- Excessive copper exposure can have adverse effects
Health Effects of Excessive Copper Exposure
Excessive copper in drinking water can lead to various health effects, including acute and chronic toxicity. Some of the potential health effects of copper exposure include:
1. Gastrointestinal Issues: Consuming water with high copper levels can cause nausea, vomiting, abdominal pain, and diarrhea.
2. Liver and Kidney Damage: Prolonged exposure to high copper levels may lead to liver and kidney damage, impairing their normal functions.
3. Central Nervous System Effects: Copper accumulation in the brain can cause neurological symptoms such as headaches, dizziness, and irritability.
4. Hematological Effects: High copper levels can interfere with the production of red blood cells, leading to anemia or other blood disorders.
5. Carcinogenic Potential: Some studies suggest that long-term exposure to high copper concentrations may increase the risk of certain types of cancers.
Monitoring and Compliance
To ensure compliance with the acceptable limit for copper in water, regular monitoring and testing of water sources are necessary. Public water supplies are typically regulated by national or regional authorities that establish guidelines and standards for water quality, including copper concentration.
Water treatment processes, such as filtration and chemical treatment, can help reduce copper levels in drinking water. Additionally, proper maintenance of plumbing systems and pipes can minimize the leaching of copper into the water supply.
Conclusion
The acceptable limit for copper in water is 0.05 mg/l, which is set to protect human health and the environment. Exceeding this limit can result in various health effects, including gastrointestinal issues, liver and kidney damage, neurological symptoms, and hematological disorders. Regular monitoring and compliance with water quality standards are essential to ensure the safety of drinking water.
The maximum allowable concentration of iron in water is
- a)1.0 ppm
- b)0.05 ppm
- c)0.3 ppm
- d)0.03 ppm
Correct answer is option 'C'. Can you explain this answer?
The maximum allowable concentration of iron in water is
a)
1.0 ppm
b)
0.05 ppm
c)
0.3 ppm
d)
0.03 ppm

|
Garima Kulkarni answered |
The maximum allowable concentration of iron in water is 0.3 ppm (parts per million). This means that the concentration of iron in water should not exceed 0.3 parts per million.
Iron is a common contaminant in water and can enter water sources through natural processes such as weathering of rocks and soil erosion, as well as through human activities such as industrial discharge and corrosion of iron pipes. While iron is an essential nutrient for the human body, high concentrations of iron in drinking water can have adverse effects on taste, odor, and appearance. It can also lead to staining of laundry, plumbing fixtures, and appliances.
To ensure the safety and quality of drinking water, regulatory agencies set limits on the concentration of various contaminants, including iron. The maximum allowable concentration of iron in water is based on scientific research and risk assessments to protect public health.
Explanation of Options:
a) 1.0 ppm: This option is not correct because the maximum allowable concentration of iron in water is lower than 1.0 ppm.
b) 0.05 ppm: This option is not correct because the maximum allowable concentration of iron in water is higher than 0.05 ppm.
c) 0.3 ppm: This option is correct because it states the maximum allowable concentration of iron in water.
d) 0.03 ppm: This option is not correct because the maximum allowable concentration of iron in water is higher than 0.03 ppm.
Regulatory agencies, such as the Environmental Protection Agency (EPA) in the United States, set the maximum allowable concentration of iron in water based on factors such as taste, odor, and visual appearance, as well as potential health effects. The EPA has established a secondary maximum contaminant level (SMCL) of 0.3 ppm for iron in drinking water. The SMCL is not enforceable, but it provides a guideline for maintaining the aesthetic quality of water.
It is important for water treatment facilities and individuals who rely on well water to regularly test their water for iron concentration and take appropriate measures if levels exceed the maximum allowable concentration. Water treatment methods such as coagulation, filtration, and ion exchange can be used to reduce iron levels in water.
In conclusion, the maximum allowable concentration of iron in water is 0.3 ppm. This limit is set to ensure the aesthetic quality of water and protect public health.
Iron is a common contaminant in water and can enter water sources through natural processes such as weathering of rocks and soil erosion, as well as through human activities such as industrial discharge and corrosion of iron pipes. While iron is an essential nutrient for the human body, high concentrations of iron in drinking water can have adverse effects on taste, odor, and appearance. It can also lead to staining of laundry, plumbing fixtures, and appliances.
To ensure the safety and quality of drinking water, regulatory agencies set limits on the concentration of various contaminants, including iron. The maximum allowable concentration of iron in water is based on scientific research and risk assessments to protect public health.
Explanation of Options:
a) 1.0 ppm: This option is not correct because the maximum allowable concentration of iron in water is lower than 1.0 ppm.
b) 0.05 ppm: This option is not correct because the maximum allowable concentration of iron in water is higher than 0.05 ppm.
c) 0.3 ppm: This option is correct because it states the maximum allowable concentration of iron in water.
d) 0.03 ppm: This option is not correct because the maximum allowable concentration of iron in water is higher than 0.03 ppm.
Regulatory agencies, such as the Environmental Protection Agency (EPA) in the United States, set the maximum allowable concentration of iron in water based on factors such as taste, odor, and visual appearance, as well as potential health effects. The EPA has established a secondary maximum contaminant level (SMCL) of 0.3 ppm for iron in drinking water. The SMCL is not enforceable, but it provides a guideline for maintaining the aesthetic quality of water.
It is important for water treatment facilities and individuals who rely on well water to regularly test their water for iron concentration and take appropriate measures if levels exceed the maximum allowable concentration. Water treatment methods such as coagulation, filtration, and ion exchange can be used to reduce iron levels in water.
In conclusion, the maximum allowable concentration of iron in water is 0.3 ppm. This limit is set to ensure the aesthetic quality of water and protect public health.
Pick up the correct statement(s)- a)turbidimeters are frequently used to measure turbidities of raw supplies
- b)turbidimeters are frequently installed on line in treatment plant, to measure turbidities of sedimented filtered waters
- c)Nephelometers are frequently used to check and measure the turbidites of final disinfected supplies
- d)Both A and B
Correct answer is option 'D'. Can you explain this answer?
Pick up the correct statement(s)
a)
turbidimeters are frequently used to measure turbidities of raw supplies
b)
turbidimeters are frequently installed on line in treatment plant, to measure turbidities of sedimented filtered waters
c)
Nephelometers are frequently used to check and measure the turbidites of final disinfected supplies
d)
Both A and B

|
Tanvi Sarkar answered |
Understanding Turbidimeters and Nephelometers
Turbidimeters and nephelometers play crucial roles in measuring water quality, particularly in assessing turbidity levels, which indicate the clarity of water.
Statement A: Turbidimeters in Raw Supplies
- Turbidimeters are indeed frequently used to measure the turbidity of raw water supplies.
- Raw water often contains suspended particles, which can affect treatment processes.
- Monitoring turbidity helps in making informed decisions about the treatment needed.
Statement B: Turbidimeters in Treatment Plants
- Turbidimeters are also commonly installed on-line in treatment plants.
- They measure the turbidity of sedimented and filtered waters to ensure that the treatment processes are effective.
- Continuous monitoring allows for real-time adjustments to optimize water quality.
Statement C: Nephelometers in Final Disinfected Supplies
- While nephelometers can be used to measure turbidity, they are not as commonly utilized for final disinfected supplies.
- Nephelometry measures scattered light to determine turbidity, making it suitable for various applications, but it is more often associated with research or laboratory settings rather than routine final checks in treatment plants.
Conclusion: The Correct Answer
- The correct answer is D: Both A and B because both statements accurately reflect the practical applications of turbidimeters in assessing water quality during different stages of treatment.
- Statement C, while true about nephelometers, does not align with the primary use cases for monitoring final disinfected waters.
Understanding these distinctions is vital for effective water treatment and ensuring compliance with quality standards.
Turbidimeters and nephelometers play crucial roles in measuring water quality, particularly in assessing turbidity levels, which indicate the clarity of water.
Statement A: Turbidimeters in Raw Supplies
- Turbidimeters are indeed frequently used to measure the turbidity of raw water supplies.
- Raw water often contains suspended particles, which can affect treatment processes.
- Monitoring turbidity helps in making informed decisions about the treatment needed.
Statement B: Turbidimeters in Treatment Plants
- Turbidimeters are also commonly installed on-line in treatment plants.
- They measure the turbidity of sedimented and filtered waters to ensure that the treatment processes are effective.
- Continuous monitoring allows for real-time adjustments to optimize water quality.
Statement C: Nephelometers in Final Disinfected Supplies
- While nephelometers can be used to measure turbidity, they are not as commonly utilized for final disinfected supplies.
- Nephelometry measures scattered light to determine turbidity, making it suitable for various applications, but it is more often associated with research or laboratory settings rather than routine final checks in treatment plants.
Conclusion: The Correct Answer
- The correct answer is D: Both A and B because both statements accurately reflect the practical applications of turbidimeters in assessing water quality during different stages of treatment.
- Statement C, while true about nephelometers, does not align with the primary use cases for monitoring final disinfected waters.
Understanding these distinctions is vital for effective water treatment and ensuring compliance with quality standards.
The commonly used indicator for measuring iron concentration in water is- a)Eriochrome black T
- b)1,10, phenanthroline
- c)Phenolphthalein
- d)Blue litmus
Correct answer is option 'B'. Can you explain this answer?
The commonly used indicator for measuring iron concentration in water is
a)
Eriochrome black T
b)
1,10, phenanthroline
c)
Phenolphthalein
d)
Blue litmus

|
Arya Menon answered |
Eriochrome black T is used to measure hardness. Phenolphthalein is used to measure alkalinity. Blue litmus is used to measure acidity of water.
The threshold odour number (TON) for a water sample of 40 ml, diluted to standard 200 ml mixture, in which odour is just barely detectable to the sense of smell, is- a)8
- b)5
- c)50
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
The threshold odour number (TON) for a water sample of 40 ml, diluted to standard 200 ml mixture, in which odour is just barely detectable to the sense of smell, is
a)
8
b)
5
c)
50
d)
None of these

|
Yash Joshi answered |
Threshold odour number represents the dilution ratio at which the odour is hardly detectable,
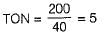
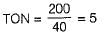
Higher quantities of copper, more than 2.5 mg/l or so, may cause diseases pertaining to- a)kidneys
- b)lungs
- c)lever
- d)arsenic
Correct answer is option 'B'. Can you explain this answer?
Higher quantities of copper, more than 2.5 mg/l or so, may cause diseases pertaining to
a)
kidneys
b)
lungs
c)
lever
d)
arsenic

|
Rounak Saini answered |
High quantities of copper are likely to affect human lungs and other respiratory organs.
What is the most common cause of acidity in water?- a)Carbon monoxide
- b)Nitrogen
- c)Hydrogen
- d)Carbon dioxide
Correct answer is option 'D'. Can you explain this answer?
What is the most common cause of acidity in water?
a)
Carbon monoxide
b)
Nitrogen
c)
Hydrogen
d)
Carbon dioxide

|
Athira Pillai answered |
CO2 reacts with water to form carbonic acid.
Which one of the following tests of water/ wastewater employs Eriochrome Black T as an indicator?- a)Hardness
- b)COD
- c)Residual chlorine
- d)DO
Correct answer is option 'A'. Can you explain this answer?
Which one of the following tests of water/ wastewater employs Eriochrome Black T as an indicator?
a)
Hardness
b)
COD
c)
Residual chlorine
d)
DO

|
Srestha Khanna answered |
The correct answer is option 'A': Hardness.
Explanation:
Eriochrome Black T is a commonly used indicator for determining water hardness. It is a synthetic dye that forms a colored complex with metal ions, particularly calcium and magnesium ions. The color change occurs when Eriochrome Black T binds to these metal ions, allowing for easy detection and quantification of hardness.
Testing water for hardness is important because it helps determine the amount of dissolved calcium and magnesium ions present. High levels of hardness can lead to scale formation in pipes and appliances, reduced efficiency of water heaters, and increased soap consumption. Thus, it is essential to monitor and control water hardness in order to avoid these issues.
There are different methods to measure water hardness, and one of them involves the use of Eriochrome Black T as an indicator. This method is known as the complexometric titration method.
Complexometric titration is a type of volumetric analysis where a known concentration of a titrant (in this case, a solution of a chelating agent such as ethylenediaminetetraacetic acid (EDTA)) is added to the water sample until the endpoint is reached. The endpoint is determined by the color change of the indicator, which occurs when all the calcium and magnesium ions are complexed with the chelating agent.
Here is how the complexometric titration method using Eriochrome Black T as an indicator works:
1. A water sample is collected and filtered to remove any suspended solids.
2. A known volume of the water sample is transferred into a titration flask.
3. A few drops of Eriochrome Black T indicator are added to the flask. The indicator forms a wine-red complex with calcium and magnesium ions.
4. A standardized solution of EDTA (titrant) is slowly added to the flask while swirling the solution. EDTA acts as a chelating agent, forming stable complexes with calcium and magnesium ions.
5. The titration is continued until the color of the solution changes from wine-red to blue. This color change indicates that all the calcium and magnesium ions have been complexed with EDTA.
6. The volume of EDTA solution required to reach the endpoint is recorded.
7. The hardness of the water sample is calculated based on the volume of EDTA solution used and the concentration of the EDTA solution.
In summary, Eriochrome Black T is used as an indicator in the complexometric titration method to determine water hardness. It forms a colored complex with calcium and magnesium ions, allowing for the endpoint of the titration to be easily detected.
Explanation:
Eriochrome Black T is a commonly used indicator for determining water hardness. It is a synthetic dye that forms a colored complex with metal ions, particularly calcium and magnesium ions. The color change occurs when Eriochrome Black T binds to these metal ions, allowing for easy detection and quantification of hardness.
Testing water for hardness is important because it helps determine the amount of dissolved calcium and magnesium ions present. High levels of hardness can lead to scale formation in pipes and appliances, reduced efficiency of water heaters, and increased soap consumption. Thus, it is essential to monitor and control water hardness in order to avoid these issues.
There are different methods to measure water hardness, and one of them involves the use of Eriochrome Black T as an indicator. This method is known as the complexometric titration method.
Complexometric titration is a type of volumetric analysis where a known concentration of a titrant (in this case, a solution of a chelating agent such as ethylenediaminetetraacetic acid (EDTA)) is added to the water sample until the endpoint is reached. The endpoint is determined by the color change of the indicator, which occurs when all the calcium and magnesium ions are complexed with the chelating agent.
Here is how the complexometric titration method using Eriochrome Black T as an indicator works:
1. A water sample is collected and filtered to remove any suspended solids.
2. A known volume of the water sample is transferred into a titration flask.
3. A few drops of Eriochrome Black T indicator are added to the flask. The indicator forms a wine-red complex with calcium and magnesium ions.
4. A standardized solution of EDTA (titrant) is slowly added to the flask while swirling the solution. EDTA acts as a chelating agent, forming stable complexes with calcium and magnesium ions.
5. The titration is continued until the color of the solution changes from wine-red to blue. This color change indicates that all the calcium and magnesium ions have been complexed with EDTA.
6. The volume of EDTA solution required to reach the endpoint is recorded.
7. The hardness of the water sample is calculated based on the volume of EDTA solution used and the concentration of the EDTA solution.
In summary, Eriochrome Black T is used as an indicator in the complexometric titration method to determine water hardness. It forms a colored complex with calcium and magnesium ions, allowing for the endpoint of the titration to be easily detected.
Blue baby disease found in infants is due to excessive _____ in drinking water.- a)Colour
- b)Sulphates
- c)Carbonates
- d)Nitrates
Correct answer is option 'D'. Can you explain this answer?
Blue baby disease found in infants is due to excessive _____ in drinking water.
a)
Colour
b)
Sulphates
c)
Carbonates
d)
Nitrates
|
|
Lavanya Menon answered |
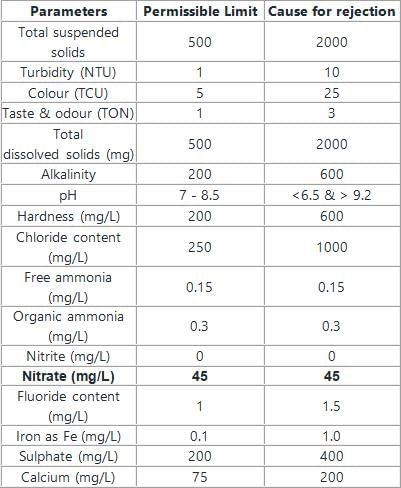
The permissible limits of various compounds are as follows:
Fluoride or fluorine deficiency is a disorder that may cause increased dental caries (or tooth decay) is the breakdown of dental tissues by the acidic products released by the bacterial fermentation of dietary carbohydrates.
Excess of nitrates is harmful to infants and causes Methemoglobinemia or Blue baby disease.
Lead in excess is toxic to many organs and tissues including heart, kidney, bones, intestines, reproductive system and nervous system. Excess lead causes anemia.

Fluoride or fluorine deficiency is a disorder that may cause increased dental caries (or tooth decay) is the breakdown of dental tissues by the acidic products released by the bacterial fermentation of dietary carbohydrates.
Excess of nitrates is harmful to infants and causes Methemoglobinemia or Blue baby disease.
Lead in excess is toxic to many organs and tissues including heart, kidney, bones, intestines, reproductive system and nervous system. Excess lead causes anemia.
Breweries and distilleries preferably require- a)hard water
- b)soft water
- c)potable water
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Breweries and distilleries preferably require
a)
hard water
b)
soft water
c)
potable water
d)
None of these

|
Ameya Deshpande answered |
The preferred water type for breweries and distilleries is hard water. This is because hard water contains higher levels of minerals such as calcium and magnesium. These minerals can have a positive impact on the taste and quality of beer and spirits.
Benefits of hard water for breweries and distilleries:
1. Enhances flavor: Hard water contains minerals that can enhance the flavor of beer and spirits, making them more complex and flavorful.
2. Improves clarity: The minerals in hard water can also help to improve the clarity of beer and spirits.
3. Consistency: The mineral content in hard water is consistent, which can help to ensure that batches of beer or spirits have a consistent taste and quality.
4. Yeast health: Hard water can also be beneficial for yeast health, as the minerals can provide the necessary nutrients for yeast growth and fermentation.
The use of soft water in breweries and distilleries is not recommended as it is low in minerals and can result in a flat taste and poor quality. Potable water, which is safe for drinking, can be used but may not provide the desired taste and quality that hard water can offer.
In conclusion, the use of hard water is preferred in breweries and distilleries due to its mineral content and positive impact on flavor, clarity, consistency, and yeast health.
Benefits of hard water for breweries and distilleries:
1. Enhances flavor: Hard water contains minerals that can enhance the flavor of beer and spirits, making them more complex and flavorful.
2. Improves clarity: The minerals in hard water can also help to improve the clarity of beer and spirits.
3. Consistency: The mineral content in hard water is consistent, which can help to ensure that batches of beer or spirits have a consistent taste and quality.
4. Yeast health: Hard water can also be beneficial for yeast health, as the minerals can provide the necessary nutrients for yeast growth and fermentation.
The use of soft water in breweries and distilleries is not recommended as it is low in minerals and can result in a flat taste and poor quality. Potable water, which is safe for drinking, can be used but may not provide the desired taste and quality that hard water can offer.
In conclusion, the use of hard water is preferred in breweries and distilleries due to its mineral content and positive impact on flavor, clarity, consistency, and yeast health.
Presence of nitrogen in a waste water sample is due to the decomposition of- a)carbohydrates
- b) proteins
- c)fats
- d)vitamins
Correct answer is option 'B'. Can you explain this answer?
Presence of nitrogen in a waste water sample is due to the decomposition of
a)
carbohydrates
b)
proteins
c)
fats
d)
vitamins

|
Navya Sarkar answered |
Nitrogen in Waste Water
Introduction:
Nitrogen is an essential element found in various organic compounds. It is commonly present in waste water due to the decomposition of organic matter. In this case, the presence of nitrogen in a waste water sample is specifically attributed to the decomposition of proteins.
Decomposition of Proteins:
Proteins are macromolecules composed of amino acids joined together by peptide bonds. When proteins undergo decomposition, they are broken down into their constituent amino acids. This process is carried out by microorganisms such as bacteria and fungi, which produce enzymes called proteases to break down proteins into smaller fragments.
Release of Nitrogen:
During the decomposition of proteins, one of the key byproducts is the release of nitrogen. Proteins contain nitrogen atoms within their amino acid structure. When proteins are broken down, these nitrogen atoms are released in the form of ammonium ions (NH4+). Ammonium ions are highly soluble in water and can be easily detected in waste water samples.
Role of Microorganisms:
Microorganisms play a crucial role in the decomposition of proteins and the release of nitrogen. Bacteria and fungi are the primary decomposers in waste water treatment systems. They secrete protease enzymes to break down proteins into smaller peptides and amino acids. These smaller molecules are then further degraded into ammonium ions, which contribute to the nitrogen content in the waste water sample.
Significance of Nitrogen in Waste Water:
The presence of nitrogen in waste water is an important parameter to consider in the context of water quality and treatment. Excessive nitrogen in waste water can lead to eutrophication, a process where excessive nutrients stimulate the growth of algae and other aquatic plants. This can result in the depletion of oxygen levels in water bodies, causing harm to aquatic life.
Conclusion:
In conclusion, the presence of nitrogen in a waste water sample is primarily due to the decomposition of proteins. Proteins contain nitrogen atoms, and when they undergo decomposition by microorganisms, nitrogen is released in the form of ammonium ions. Monitoring and controlling nitrogen levels in waste water is crucial for effective wastewater treatment and preserving water quality.
Introduction:
Nitrogen is an essential element found in various organic compounds. It is commonly present in waste water due to the decomposition of organic matter. In this case, the presence of nitrogen in a waste water sample is specifically attributed to the decomposition of proteins.
Decomposition of Proteins:
Proteins are macromolecules composed of amino acids joined together by peptide bonds. When proteins undergo decomposition, they are broken down into their constituent amino acids. This process is carried out by microorganisms such as bacteria and fungi, which produce enzymes called proteases to break down proteins into smaller fragments.
Release of Nitrogen:
During the decomposition of proteins, one of the key byproducts is the release of nitrogen. Proteins contain nitrogen atoms within their amino acid structure. When proteins are broken down, these nitrogen atoms are released in the form of ammonium ions (NH4+). Ammonium ions are highly soluble in water and can be easily detected in waste water samples.
Role of Microorganisms:
Microorganisms play a crucial role in the decomposition of proteins and the release of nitrogen. Bacteria and fungi are the primary decomposers in waste water treatment systems. They secrete protease enzymes to break down proteins into smaller peptides and amino acids. These smaller molecules are then further degraded into ammonium ions, which contribute to the nitrogen content in the waste water sample.
Significance of Nitrogen in Waste Water:
The presence of nitrogen in waste water is an important parameter to consider in the context of water quality and treatment. Excessive nitrogen in waste water can lead to eutrophication, a process where excessive nutrients stimulate the growth of algae and other aquatic plants. This can result in the depletion of oxygen levels in water bodies, causing harm to aquatic life.
Conclusion:
In conclusion, the presence of nitrogen in a waste water sample is primarily due to the decomposition of proteins. Proteins contain nitrogen atoms, and when they undergo decomposition by microorganisms, nitrogen is released in the form of ammonium ions. Monitoring and controlling nitrogen levels in waste water is crucial for effective wastewater treatment and preserving water quality.
Match List-I (Parameter) with List-ll (Impact) and select the correct answer using the codes given below the lists: List-l
A. Excess sulphates
B. Lack of iodide
C. Excess hardness
D. Excess dissolved oxygenList-ll
1. Greater soap consumption
2. Laxative effect
3. Goitre
4. Corrosion of pipesCodes:
A B C D
(a) 2 1 3 4
(b) 4 3 1 2
(c) 2 3 1 4
(d) 4 1 3 2- a)a
- b)b
- c)c
- d)d
Correct answer is option 'C'. Can you explain this answer?
Match List-I (Parameter) with List-ll (Impact) and select the correct answer using the codes given below the lists: List-l
A. Excess sulphates
B. Lack of iodide
C. Excess hardness
D. Excess dissolved oxygen
A. Excess sulphates
B. Lack of iodide
C. Excess hardness
D. Excess dissolved oxygen
List-ll
1. Greater soap consumption
2. Laxative effect
3. Goitre
4. Corrosion of pipes
1. Greater soap consumption
2. Laxative effect
3. Goitre
4. Corrosion of pipes
Codes:
A B C D
(a) 2 1 3 4
(b) 4 3 1 2
(c) 2 3 1 4
(d) 4 1 3 2
A B C D
(a) 2 1 3 4
(b) 4 3 1 2
(c) 2 3 1 4
(d) 4 1 3 2
a)
a
b)
b
c)
c
d)
d

|
Ashwin Kulkarni answered |
Parameter: Excess sulphates
Impact: Greater soap consumption
Parameter: Lack of iodide
Impact: Goitre
Parameter: Excess hardness
Impact: Laxative effect
Parameter: Excess dissolved oxygen
Impact: Corrosion of pipes
Explanation:
Excess sulphates in water can lead to greater soap consumption as they form insoluble compounds with soap, making it less effective. Lack of iodide in water can cause goitre, which is a swelling of the thyroid gland in the neck. Excess hardness in water can have a laxative effect on the body, causing diarrhea. Excess dissolved oxygen in water can cause corrosion of pipes, which can lead to leaks and contamination of the water supply.
Therefore, the correct answer is option C, with the matching parameters and impacts being:
- Excess sulphates -> Greater soap consumption
- Lack of iodide -> Goitre
- Excess hardness -> Laxative effect
- Excess dissolved oxygen -> Corrosion of pipes
Impact: Greater soap consumption
Parameter: Lack of iodide
Impact: Goitre
Parameter: Excess hardness
Impact: Laxative effect
Parameter: Excess dissolved oxygen
Impact: Corrosion of pipes
Explanation:
Excess sulphates in water can lead to greater soap consumption as they form insoluble compounds with soap, making it less effective. Lack of iodide in water can cause goitre, which is a swelling of the thyroid gland in the neck. Excess hardness in water can have a laxative effect on the body, causing diarrhea. Excess dissolved oxygen in water can cause corrosion of pipes, which can lead to leaks and contamination of the water supply.
Therefore, the correct answer is option C, with the matching parameters and impacts being:
- Excess sulphates -> Greater soap consumption
- Lack of iodide -> Goitre
- Excess hardness -> Laxative effect
- Excess dissolved oxygen -> Corrosion of pipes
Chapter doubts & questions for Quality Parameters of Water - Environmental Engineering 2025 is part of Civil Engineering (CE) exam preparation. The chapters have been prepared according to the Civil Engineering (CE) exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Civil Engineering (CE) 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Quality Parameters of Water - Environmental Engineering in English & Hindi are available as part of Civil Engineering (CE) exam.
Download more important topics, notes, lectures and mock test series for Civil Engineering (CE) Exam by signing up for free.
Environmental Engineering
14 videos|142 docs|98 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily









