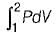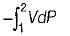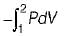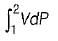All Exams >
Mechanical Engineering >
Thermodynamics >
All Questions
All questions of First Law of Thermodynamics for Mechanical Engineering Exam
The net work done for the closed system shown in the given pressure-volume diagram is
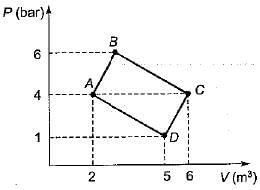
- a)600 kN-m
- b)1000 kN-m
- c)900 kN-m
- d)700kN-m
Correct answer is option 'B'. Can you explain this answer?
The net work done for the closed system shown in the given pressure-volume diagram is


a)
600 kN-m
b)
1000 kN-m
c)
900 kN-m
d)
700kN-m

|
Manish Aggarwal answered |
Correct Answer :- B
Explanation : AC = 4 m3.
ABC = 2, ACD = 3
Work done = Area of ABC + Area of ACD
= 1/2 * 2 * 4 + 1/2 * 3 * 4
= 1000 kN-m.
A stationary mass of gas is compressed without friction from an initial state of 0.3 m3 and 1 MPa to a final state of 0.15 m3 and 1 MPa, the pressure remaining constant during the process. There is a transfer of 40 kJ of heat from the gas during the process. What is the change in internal energy of the gas?- a)-5 kJ
- b)+25 kJ
- c)-25 kJ
- d)+15 kJ
Correct answer is option 'C'. Can you explain this answer?
A stationary mass of gas is compressed without friction from an initial state of 0.3 m3 and 1 MPa to a final state of 0.15 m3 and 1 MPa, the pressure remaining constant during the process. There is a transfer of 40 kJ of heat from the gas during the process. What is the change in internal energy of the gas?
a)
-5 kJ
b)
+25 kJ
c)
-25 kJ
d)
+15 kJ
|
|
Shruti Bose answered |
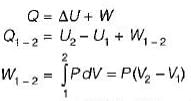
= 0.1 χ 106 (0.15-0.3)
= -15Kj
 -25Kj
-25KjEnergy is added to 5 kg of air with a paddle wheel so that ΔT = 100°C while P = const. in an insulated container. The paddle-wheel work is- a)502.5 kJ
- b)482 kJ
- c)412 kJ
- d)358 kJ
Correct answer is option 'D'. Can you explain this answer?
Energy is added to 5 kg of air with a paddle wheel so that ΔT = 100°C while P = const. in an insulated container. The paddle-wheel work is
a)
502.5 kJ
b)
482 kJ
c)
412 kJ
d)
358 kJ

|
Gate Funda answered |
To determine the paddle-wheel work done on 5 kg of air with a temperature increase of 100°C at constant pressure in an insulated container, we use thermodynamic principles.
- Identify the Process:
- Since the container is insulated, there's no heat transfer (Q = 0).
- The first law of thermodynamics simplifies to ΔU = W, where W is the work done by the paddle wheel.
- Internal Energy Change Calculation:
- Air can be treated as an ideal diatomic gas.
- Specific heat at constant volume for a diatomic gas, Cv = (5/2)R = 20.785 J/mol·K.
- Molar mass of air ≈ 28.97 g/mol → Moles of air, n = 5000 g / 28.97 g/mol ≈ 172.6 mol.
- ΔU = n × Cv × ΔT = 172.6 × 20.785 J/K × 100 K ≈ 358,800 J or 358.8 kJ.
- Conclusion: The work done by the paddle wheel equals the internal energy change (ΔU), resulting in approximately 358 kJ.
Thus, the correct answer is option D: 358 kJ.
A closed system undergoes a process 1-2 for which the values of Q1-2 and W1-2 are +20 kJ and +50 kJ respectively. If the system is returned to state 1 and Q2-1 is - 10 kJ what is the value of work W2-1- a)+20 kJ
- b)-40 kJ
- c)-80 kJ
- d)+40 kJ
Correct answer is option 'B'. Can you explain this answer?
A closed system undergoes a process 1-2 for which the values of Q1-2 and W1-2 are +20 kJ and +50 kJ respectively. If the system is returned to state 1 and Q2-1 is - 10 kJ what is the value of work W2-1
a)
+20 kJ
b)
-40 kJ
c)
-80 kJ
d)
+40 kJ
|
|
Jhanvi Datta answered |
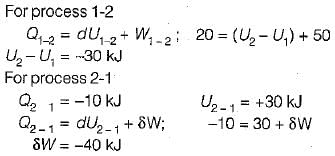
If steam is throttled its- a)pressure and enthalpy remain unchanged
- b)temperature and entropy remain unchanged
- c)enthalpy remains unchanged but the other property change
- d)enthalpy remains unchanged but pressure may or may not chan
Correct answer is option 'C'. Can you explain this answer?
If steam is throttled its
a)
pressure and enthalpy remain unchanged
b)
temperature and entropy remain unchanged
c)
enthalpy remains unchanged but the other property change
d)
enthalpy remains unchanged but pressure may or may not chan

|
Rajat Patel answered |
If steam is throttled , its enthalpy remains constant and pressure drop takes place
An engine is tested by means of a water brake at 1000 rpm. The measured torque-of the engine is 10000 Nm and the water consumption of the brake is 0.5 m3/s, its inlet temperature being 25°C. Assuming that the whole of the engine power is ultimately transferred into heat which is absorbed by the cooling water, what is the water temperature at exit?- a)22.5°C
- b)23.5°C
- c)24.5°C
- d)25.5°C
Correct answer is option 'D'. Can you explain this answer?
An engine is tested by means of a water brake at 1000 rpm. The measured torque-of the engine is 10000 Nm and the water consumption of the brake is 0.5 m3/s, its inlet temperature being 25°C. Assuming that the whole of the engine power is ultimately transferred into heat which is absorbed by the cooling water, what is the water temperature at exit?
a)
22.5°C
b)
23.5°C
c)
24.5°C
d)
25.5°C
|
|
Stuti Mishra answered |
Engine power,
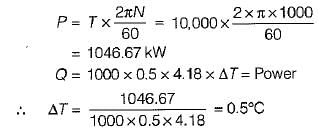
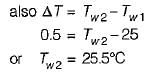
A PMM1 is- a)A thermodynamic machine
- b)A hypothetical machine
- c)A real machine
- d)A hypothetical machine whose operation would violate the first law of thermodynamic
Correct answer is option 'D'. Can you explain this answer?
A PMM1 is
a)
A thermodynamic machine
b)
A hypothetical machine
c)
A real machine
d)
A hypothetical machine whose operation would violate the first law of thermodynamic

|
Meghana Desai answered |
There can be no machine which would continuously supply mechanical work without some other form of energy disappearing simultaneously. It is fictitious machine.
During a thermodynamic process, 84 kj of heat flows into the system and the work done by the system is 32 kJ. The increase in internal energy of the system is- a)+52 kJ
- b)-52 kj
- c)+116 kJ
- d)-116 kJ
Correct answer is option 'A'. Can you explain this answer?
During a thermodynamic process, 84 kj of heat flows into the system and the work done by the system is 32 kJ. The increase in internal energy of the system is
a)
+52 kJ
b)
-52 kj
c)
+116 kJ
d)
-116 kJ
|
|
Pritam Jain answered |
From first law of thermodynamics
δQ = dU+δW
84 = dU +32
dU = 52kJ
δQ = dU+δW
84 = dU +32
dU = 52kJ
The state of an ideal gas is changed from (T1, P1) to (T2, P2) in a constant volume process. To calculate the change in enthalpy (Δh) ail of the following properties/variables are required.
- a)Cv,P1, P2
- b)Cp, T1, T2
- c)Cp, T1, T2, P1, P2
- d)Cv, P1 P2, T1, T2
Correct answer is option 'B'. Can you explain this answer?
The state of an ideal gas is changed from (T1, P1) to (T2, P2) in a constant volume process. To calculate the change in enthalpy (Δh) ail of the following properties/variables are required.
a)
Cv,P1, P2
b)
Cp, T1, T2
c)
Cp, T1, T2, P1, P2
d)
Cv, P1 P2, T1, T2

|
Anisha Chakraborty answered |
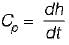 Calculation of Change in Enthalpy (Δh) in Constant Volume Process
Calculation of Change in Enthalpy (Δh) in Constant Volume Process- Properties/Variables Required:
- Cp (specific heat at constant pressure)
- Initial temperature (T1)
- Final temperature (T2)
- In a constant volume process, the enthalpy change (Δh) is equal to the heat added to the system. The formula for calculating the change in enthalpy is Δh = Cp * (T2 - T1).
- Cp is the specific heat at constant pressure, which represents the amount of heat required to raise the temperature of one unit mass of a substance by one degree Celsius at constant pressure.
- We need the initial temperature (T1) and final temperature (T2) to calculate the change in enthalpy, as the enthalpy change is directly proportional to the temperature change.
- Therefore, the properties/variables required to calculate the change in enthalpy (Δh) in a constant volume process are Cp, T1, and T2.
Internal energy is defined by- a)Zeroth law of thermodynamics
- b)First law of thermodynamics
- c)Second law of thermodynamics
- d)Law of entrop
Correct answer is option 'B'. Can you explain this answer?
Internal energy is defined by
a)
Zeroth law of thermodynamics
b)
First law of thermodynamics
c)
Second law of thermodynamics
d)
Law of entrop

|
Akanksha Mehta answered |
Zeroth law of thermodynamics — concept of temperature
First law of thermodynamics — concept of internal energy
Second law of thermodynamics — concept of entropy.
First law of thermodynamics — concept of internal energy
Second law of thermodynamics — concept of entropy.
The first law of thermodynamics is the law of- a)conservation of mass
- b)conservation of energy
- c)conservation of momentum
- d)conservation of temperature
Correct answer is option 'B'. Can you explain this answer?
The first law of thermodynamics is the law of
a)
conservation of mass
b)
conservation of energy
c)
conservation of momentum
d)
conservation of temperature
|
|
Nisha Singh answered |
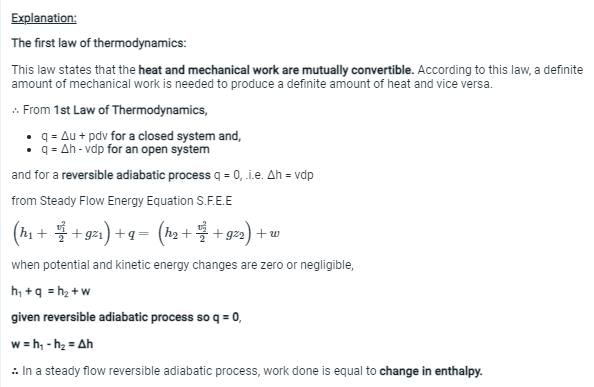
Explanation:
The first law of thermodynamics is also known as the law of conservation of energy. It states that energy cannot be created or destroyed, only converted from one form to another or transferred from one system to another. This law is a fundamental principle of physics and applies to all systems, including mechanical, electrical, chemical, and thermal systems.
The law of conservation of energy has several important implications:
1. Energy is a scalar quantity: The total energy of a system is the sum of the energies of its individual components. This means that energy can be added or removed from a system in different ways, but the total energy of the system remains constant.
2. Energy can be converted from one form to another: Any form of energy can be converted into any other form of energy. For example, mechanical energy can be converted into electrical energy, or thermal energy can be converted into kinetic energy.
3. Energy can be transferred from one system to another: Energy can be transferred between different systems in the form of work or heat. For example, when a hot object is placed in contact with a cold object, heat flows from the hot object to the cold object until they reach thermal equilibrium.
4. The first law of thermodynamics applies to closed systems: The law of conservation of energy applies to closed systems, which are systems that do not exchange matter or energy with their surroundings. In other words, the total energy of a closed system remains constant over time.
In summary, the first law of thermodynamics is the law of conservation of energy, which states that energy cannot be created or destroyed, only converted from one form to another or transferred from one system to another. This law has several important implications for understanding the behavior of physical systems, and it applies to all forms of energy, including mechanical, electrical, chemical, and thermal energy.
The first law of thermodynamics is also known as the law of conservation of energy. It states that energy cannot be created or destroyed, only converted from one form to another or transferred from one system to another. This law is a fundamental principle of physics and applies to all systems, including mechanical, electrical, chemical, and thermal systems.
The law of conservation of energy has several important implications:
1. Energy is a scalar quantity: The total energy of a system is the sum of the energies of its individual components. This means that energy can be added or removed from a system in different ways, but the total energy of the system remains constant.
2. Energy can be converted from one form to another: Any form of energy can be converted into any other form of energy. For example, mechanical energy can be converted into electrical energy, or thermal energy can be converted into kinetic energy.
3. Energy can be transferred from one system to another: Energy can be transferred between different systems in the form of work or heat. For example, when a hot object is placed in contact with a cold object, heat flows from the hot object to the cold object until they reach thermal equilibrium.
4. The first law of thermodynamics applies to closed systems: The law of conservation of energy applies to closed systems, which are systems that do not exchange matter or energy with their surroundings. In other words, the total energy of a closed system remains constant over time.
In summary, the first law of thermodynamics is the law of conservation of energy, which states that energy cannot be created or destroyed, only converted from one form to another or transferred from one system to another. This law has several important implications for understanding the behavior of physical systems, and it applies to all forms of energy, including mechanical, electrical, chemical, and thermal energy.
The specific heat at constant pressure for an ideal gas is given by
cp = 0.9 + (2.7 x 10-4) T (kJ/kgK)
Where T is in kelvin. The change in enthalpy for this ideal gas undergoing a process in which the temperature changes from 27°C to 127°C is most nearly.- a)90 kJ/kg
- b)108.9 kJ/kg
- c)99.5 kJ/kg
- d)105.2 kJ/kg
Correct answer is option 'C'. Can you explain this answer?
The specific heat at constant pressure for an ideal gas is given by
cp = 0.9 + (2.7 x 10-4) T (kJ/kgK)
Where T is in kelvin. The change in enthalpy for this ideal gas undergoing a process in which the temperature changes from 27°C to 127°C is most nearly.
cp = 0.9 + (2.7 x 10-4) T (kJ/kgK)
Where T is in kelvin. The change in enthalpy for this ideal gas undergoing a process in which the temperature changes from 27°C to 127°C is most nearly.
a)
90 kJ/kg
b)
108.9 kJ/kg
c)
99.5 kJ/kg
d)
105.2 kJ/kg

|
Lekshmi Das answered |

What is the temperature rise after 5 minutes in the below volume?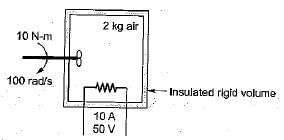
- a)423°C
- b)378°C
- c)313
- d)287°
Correct answer is option 'C'. Can you explain this answer?
What is the temperature rise after 5 minutes in the below volume?

a)
423°C
b)
378°C
c)
313
d)
287°

|
Nivas Roxx answered |
What is the temperature rise after 5 minutes in the below volume? a)423�C b)378�C c)313 d)287� Correct answer is option 'C'. Can you explain this answer?
Read more at:
Consider following comparative points of S.F.E.E with Bernoulli's equation:
1. These two have several terms in common.
2. Both Bernoulli’s equation and S.F.E.E is valid for viscous compressible fluids.
3. The Bernoulli’s equation is a special limiting case of S.F.E.EWhich of the above is/are correct?- a)1 only
- b)1 and 3
- c)1 and 2
- d)1, 2 and 3
Correct answer is option 'B'. Can you explain this answer?
Consider following comparative points of S.F.E.E with Bernoulli's equation:
1. These two have several terms in common.
2. Both Bernoulli’s equation and S.F.E.E is valid for viscous compressible fluids.
3. The Bernoulli’s equation is a special limiting case of S.F.E.E
1. These two have several terms in common.
2. Both Bernoulli’s equation and S.F.E.E is valid for viscous compressible fluids.
3. The Bernoulli’s equation is a special limiting case of S.F.E.E
Which of the above is/are correct?
a)
1 only
b)
1 and 3
c)
1 and 2
d)
1, 2 and 3

|
Baishali Chopra answered |
Bernoulli’s equation valid for frictionless incompressible fluids. S.F.E.E. valid for viscous compressible fluids.
The term Δh in a control volume equation Q - W = Δh- a)Accounts for the rate of change in energy of the control volume.
- b)Represents the rate of change of energy between the inlet and outlet.
- c)Is often neglected in control-volume applications?
- d)Includes the work rate due to the pressure forces.
Correct answer is option 'D'. Can you explain this answer?
The term Δh in a control volume equation Q - W = Δh
a)
Accounts for the rate of change in energy of the control volume.
b)
Represents the rate of change of energy between the inlet and outlet.
c)
Is often neglected in control-volume applications?
d)
Includes the work rate due to the pressure forces.
|
|
Pritam Das answered |
S.F.E.E:
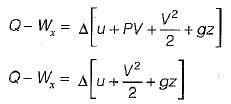
Δh accounts for internal energy and pressure forces
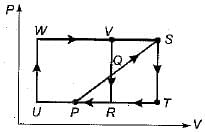
Two ideal heat engine cycles are represented in the given figure. Assume VQ = QR, PQ = QS and UP = PR= RT. If the work interaction for the rectangular cycle (WVRU) is 48 Nm, then the work interaction for the other cycle PST is
- a)12Nm
- b)18Nm
- c)24 Nm
- d)36 Nm
Correct answer is option 'C'. Can you explain this answer?
Two ideal heat engine cycles are represented in the given figure. Assume VQ = QR, PQ = QS and UP = PR= RT. If the work interaction for the rectangular cycle (WVRU) is 48 Nm, then the work interaction for the other cycle PST is


a)
12Nm
b)
18Nm
c)
24 Nm
d)
36 Nm

|
Prakhar Dwivedi answered |
Since in P-V diagram, Area=Workdone.
Tringles PQR & QVS are conguent.
Work(PST)= Work (PQR) + Work(QSTR)
Work(PST)= Work (QVS) + Work(QSTR)
Work(PST)= Area (QVS + QSTR)
Work(PST)= Area (VSTR) = 0.5 * Area (WVRU)
( Since UR =2*RT)
Work(PST)= 0.5*48= 24Nm.
Tringles PQR & QVS are conguent.
Work(PST)= Work (PQR) + Work(QSTR)
Work(PST)= Work (QVS) + Work(QSTR)
Work(PST)= Area (QVS + QSTR)
Work(PST)= Area (VSTR) = 0.5 * Area (WVRU)
( Since UR =2*RT)
Work(PST)= 0.5*48= 24Nm.
In a reversible isothermal expansion process fluid expands from 10 bar and 2 m3 to 2 bar and 10 m3. During the process the heat supplied is at the rate of 100 kW. What is the rate of work done during the proces- a)20 kW
- b)35 kW
- c)80 kW
- d)100 kW
Correct answer is option 'D'. Can you explain this answer?
In a reversible isothermal expansion process fluid expands from 10 bar and 2 m3 to 2 bar and 10 m3. During the process the heat supplied is at the rate of 100 kW. What is the rate of work done during the proces
a)
20 kW
b)
35 kW
c)
80 kW
d)
100 kW
|
|
Rajat Khanna answered |
Note that in case of reversible isothermal expansion change in internal energy is zero hence
δQ = δW= 100 kW
δQ = δW= 100 kW
What reaction takes place during photosynthesis? - a)Exothermic reaction
- b)Redox reaction
- c)Endothermic reaction
- d)Combustion reaction
Correct answer is option 'C'. Can you explain this answer?
What reaction takes place during photosynthesis?
a)
Exothermic reaction
b)
Redox reaction
c)
Endothermic reaction
d)
Combustion reaction
|
|
Neha Joshi answered |
Photosynthesis takes place by absorbing heat and energy from the surroundings. Since, endothermic reaction is a reaction in which the system absorbs heat from its surroundings, the reaction that takes place during photosynthesis is an endothermic reaction.
According to first law of thermodynamics- a)total internal energy of a system during a process remains constant
- b)total energy of a system remains constant
- c)work done by a system is equal to the heat transferred by the system
- d)internal energy, enthalpy and entropy during a process remains constant hence it is an Isochoric process.
Correct answer is option 'B'. Can you explain this answer?
According to first law of thermodynamics
a)
total internal energy of a system during a process remains constant
b)
total energy of a system remains constant
c)
work done by a system is equal to the heat transferred by the system
d)
internal energy, enthalpy and entropy during a process remains constant hence it is an Isochoric process.

|
Anisha Chakraborty answered |
Since first law of thermodynamics defined as law of conservation of energy hence total energy of a system remains constant.
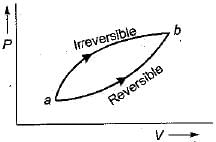
For the two paths as shown in the figure, one reversible and one irreversible, to change the state of the system from a to b.
- a)ΔU, Q, W are same
- b)ΔU is same
- c)Q, W are same
- d)ΔU Q are different
Correct answer is option 'B'. Can you explain this answer?
For the two paths as shown in the figure, one reversible and one irreversible, to change the state of the system from a to b.


a)
ΔU, Q, W are same
b)
ΔU is same
c)
Q, W are same
d)
ΔU Q are different
|
|
Sanvi Kapoor answered |
ΔU is a point function and it is independent of the path followed.
First law of thermodynamics is valid for- a)all processes
- b)only reversible processe
- c)only cyclic processes
- d)only cyclic processes that are carried out reversibly
Correct answer is option 'A'. Can you explain this answer?
First law of thermodynamics is valid for
a)
all processes
b)
only reversible processe
c)
only cyclic processes
d)
only cyclic processes that are carried out reversibly
|
|
Anshul Sharma answered |
Explanation:
The first law of thermodynamics is also known as the law of conservation of energy. It states that energy cannot be created or destroyed, only transferred or converted from one form to another. This law is valid for all processes, whether they are reversible, irreversible, or cyclic.
Validity:
The first law of thermodynamics is universally applicable and valid for all processes, including:
All Processes: The first law of thermodynamics is valid for all processes, whether they are reversible, irreversible or cyclic.
Reversible Processes: The first law of thermodynamics is valid for reversible processes, where the system and surroundings can be brought back to their initial state after the process is completed.
Irreversible Processes: The first law of thermodynamics is valid for irreversible processes, where energy is lost due to friction, heat transfer or other factors.
Cyclic Processes: The first law of thermodynamics is valid for cyclic processes, where the system returns to its initial state after a series of changes.
Conclusion:
In conclusion, the first law of thermodynamics is a fundamental principle that is universally applicable and valid for all processes, whether they are reversible, irreversible or cyclic. This law plays a crucial role in understanding the behavior of energy in various physical systems and processes.
The first law of thermodynamics is also known as the law of conservation of energy. It states that energy cannot be created or destroyed, only transferred or converted from one form to another. This law is valid for all processes, whether they are reversible, irreversible, or cyclic.
Validity:
The first law of thermodynamics is universally applicable and valid for all processes, including:
All Processes: The first law of thermodynamics is valid for all processes, whether they are reversible, irreversible or cyclic.
Reversible Processes: The first law of thermodynamics is valid for reversible processes, where the system and surroundings can be brought back to their initial state after the process is completed.
Irreversible Processes: The first law of thermodynamics is valid for irreversible processes, where energy is lost due to friction, heat transfer or other factors.
Cyclic Processes: The first law of thermodynamics is valid for cyclic processes, where the system returns to its initial state after a series of changes.
Conclusion:
In conclusion, the first law of thermodynamics is a fundamental principle that is universally applicable and valid for all processes, whether they are reversible, irreversible or cyclic. This law plays a crucial role in understanding the behavior of energy in various physical systems and processes.
Given that along the path 1-2-3 a system absorbs 100 kJ as heat and does 60 kJ work while along the path 1 -4-3 it does 20 kJ work (see figure given). The heat absorbed during the cycle 1-4-3 is
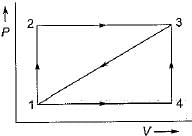
- a)-140 kJ
- b)-80 kJ
- c)-40 kJ
- d)60 kJ
Correct answer is option 'D'. Can you explain this answer?
Given that along the path 1-2-3 a system absorbs 100 kJ as heat and does 60 kJ work while along the path 1 -4-3 it does 20 kJ work (see figure given). The heat absorbed during the cycle 1-4-3 is


a)
-140 kJ
b)
-80 kJ
c)
-40 kJ
d)
60 kJ

|
Navya Kaur answered |
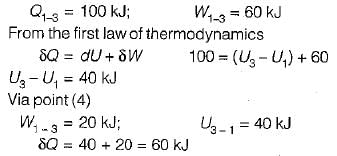
Neglecting changes in potential and kinetic energies, the shaft work during a steady flow process is given by- a)
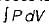
- b)
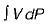
- c)P v
- d)-Δh
Correct answer is option 'B'. Can you explain this answer?
Neglecting changes in potential and kinetic energies, the shaft work during a steady flow process is given by
a)
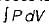
b)
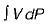
c)
P v
d)
-Δh
|
|
Aditya Chavan answered |
Work in steady flow process = 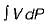
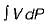
Work in non-flow process = 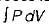
Consider the following statements about steady flow process:
1. The rate of flow of mass energy across the control surface are constant.
2. Thermodynamic properties vary along space as well as time coordinates.
3. Any thermodynamic property will have a fixed value at a particular location and will not alter with time.Which of the above are correct?- a)1 and 2
- b)1 and 3
- c)2 and 3
- d)1,2 and 3
Correct answer is option 'B'. Can you explain this answer?
Consider the following statements about steady flow process:
1. The rate of flow of mass energy across the control surface are constant.
2. Thermodynamic properties vary along space as well as time coordinates.
3. Any thermodynamic property will have a fixed value at a particular location and will not alter with time.
1. The rate of flow of mass energy across the control surface are constant.
2. Thermodynamic properties vary along space as well as time coordinates.
3. Any thermodynamic property will have a fixed value at a particular location and will not alter with time.
Which of the above are correct?
a)
1 and 2
b)
1 and 3
c)
2 and 3
d)
1,2 and 3

|
Anshul Chakraborty answered |
Thermodynamic properties may vary along space coordinates but do not vary with time.
Compressed air coming out from a punctured football- a)becomes hotter
- b)becomes cooler
- c)remains at same temperature
- d)attains atmospheric temperatur
Correct answer is option 'B'. Can you explain this answer?
Compressed air coming out from a punctured football
a)
becomes hotter
b)
becomes cooler
c)
remains at same temperature
d)
attains atmospheric temperatur

|
Mihir Kulkarni answered |
Explanation:
When a football is punctured and the compressed air inside is released, the air undergoes a process known as adiabatic expansion. This means that the air expands rapidly without exchanging heat with its surroundings. As a result of this expansion, several things happen:
1. Decrease in Pressure:
As the air escapes from the punctured football, the pressure inside the ball decreases. According to the ideal gas law, when the pressure of a gas decreases, its temperature also decreases. This is known as the Joule-Thomson effect.
2. Work Done by the Air:
The escaping air does work on the surrounding atmosphere as it expands. This work done by the air reduces its internal energy and therefore its temperature.
3. Adiabatic Cooling:
During adiabatic expansion, the air molecules move further apart, resulting in a decrease in temperature. This is because the molecules have to do work against the surrounding pressure to expand. The energy required for this work comes from the internal energy of the air, causing a decrease in temperature.
4. Cooler Air:
As a result of the adiabatic expansion, the compressed air coming out from a punctured football becomes cooler than the surrounding air. Therefore, option 'b' (becomes cooler) is the correct answer.
5. Atmospheric Temperature:
The air coming out from the punctured football will not immediately attain atmospheric temperature. It will take some time for the air to mix and reach thermal equilibrium with the surrounding atmosphere.
In conclusion, when a football is punctured and the compressed air inside is released, the air undergoes adiabatic expansion, which causes it to become cooler than the surrounding air.
When a football is punctured and the compressed air inside is released, the air undergoes a process known as adiabatic expansion. This means that the air expands rapidly without exchanging heat with its surroundings. As a result of this expansion, several things happen:
1. Decrease in Pressure:
As the air escapes from the punctured football, the pressure inside the ball decreases. According to the ideal gas law, when the pressure of a gas decreases, its temperature also decreases. This is known as the Joule-Thomson effect.
2. Work Done by the Air:
The escaping air does work on the surrounding atmosphere as it expands. This work done by the air reduces its internal energy and therefore its temperature.
3. Adiabatic Cooling:
During adiabatic expansion, the air molecules move further apart, resulting in a decrease in temperature. This is because the molecules have to do work against the surrounding pressure to expand. The energy required for this work comes from the internal energy of the air, causing a decrease in temperature.
4. Cooler Air:
As a result of the adiabatic expansion, the compressed air coming out from a punctured football becomes cooler than the surrounding air. Therefore, option 'b' (becomes cooler) is the correct answer.
5. Atmospheric Temperature:
The air coming out from the punctured football will not immediately attain atmospheric temperature. It will take some time for the air to mix and reach thermal equilibrium with the surrounding atmosphere.
In conclusion, when a football is punctured and the compressed air inside is released, the air undergoes adiabatic expansion, which causes it to become cooler than the surrounding air.
The network output for the cycle 1-2-3-4 -5-6-1 shown in figure is
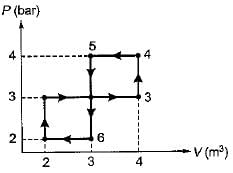
- a)200 kJ
- b)1200 kJ
- c)0 kJ
- d)1000 kJ
Correct answer is option 'C'. Can you explain this answer?
The network output for the cycle 1-2-3-4 -5-6-1 shown in figure is


a)
200 kJ
b)
1200 kJ
c)
0 kJ
d)
1000 kJ

|
Vineet Badekar answered |
Just sum up all the areas under curves and you will get the net work done

Key concept in analyzing the filling of an evacuated tank is- a)the mass flow rate in the tank remains constant
- b)the enthalpy across the valve remains constant
- c)the internal energy in the tank remains constant
- d)the temperature in the tank remains constant
Correct answer is option 'B'. Can you explain this answer?
Key concept in analyzing the filling of an evacuated tank is
a)
the mass flow rate in the tank remains constant
b)
the enthalpy across the valve remains constant
c)
the internal energy in the tank remains constant
d)
the temperature in the tank remains constant

|
Ishaan Kulkarni answered |
Analyzing the Filling of an Evacuated Tank
Key Concept: Enthalpy across the valve remains constant
When an evacuated tank is filled with a fluid, it is important to consider the thermodynamic properties of the fluid as well as the tank. One key concept to consider is the enthalpy across the valve. Enthalpy is a measure of the total energy of a system, including its internal energy and the energy required to perform work. In the case of filling an evacuated tank, the enthalpy of the fluid is important because it will affect the temperature and pressure of the fluid as it enters the tank.
Constant Enthalpy
The enthalpy across the valve must remain constant during the filling process. This is because any change in enthalpy will result in a change in temperature and pressure of the fluid, which can affect the performance of the tank and the safety of the system. If the enthalpy changes, there is a risk that the tank could become over-pressurized, which can lead to leaks or even explosions.
Mass Flow Rate
While the mass flow rate of the fluid is also important, it is not the key concept in analyzing the filling of an evacuated tank. The mass flow rate can affect the filling time and the performance of the tank, but it does not directly impact the thermodynamic properties of the fluid or the tank.
Internal Energy
The internal energy in the tank is also important, but it is not the key concept in analyzing the filling of an evacuated tank. The internal energy of the tank will change as it is filled, but this is a secondary effect that is influenced by the filling process rather than a key concept that must be considered.
Temperature
Similarly, the temperature in the tank is important, but it is not the key concept in analyzing the filling of an evacuated tank. The temperature of the fluid will change as it enters the tank, but this is a secondary effect that is influenced by the filling process rather than a key concept that must be considered.
Conclusion
In conclusion, the key concept in analyzing the filling of an evacuated tank is the enthalpy across the valve. This is because any changes in enthalpy can affect the temperature and pressure of the fluid, which can impact the performance and safety of the tank.
Key Concept: Enthalpy across the valve remains constant
When an evacuated tank is filled with a fluid, it is important to consider the thermodynamic properties of the fluid as well as the tank. One key concept to consider is the enthalpy across the valve. Enthalpy is a measure of the total energy of a system, including its internal energy and the energy required to perform work. In the case of filling an evacuated tank, the enthalpy of the fluid is important because it will affect the temperature and pressure of the fluid as it enters the tank.
Constant Enthalpy
The enthalpy across the valve must remain constant during the filling process. This is because any change in enthalpy will result in a change in temperature and pressure of the fluid, which can affect the performance of the tank and the safety of the system. If the enthalpy changes, there is a risk that the tank could become over-pressurized, which can lead to leaks or even explosions.
Mass Flow Rate
While the mass flow rate of the fluid is also important, it is not the key concept in analyzing the filling of an evacuated tank. The mass flow rate can affect the filling time and the performance of the tank, but it does not directly impact the thermodynamic properties of the fluid or the tank.
Internal Energy
The internal energy in the tank is also important, but it is not the key concept in analyzing the filling of an evacuated tank. The internal energy of the tank will change as it is filled, but this is a secondary effect that is influenced by the filling process rather than a key concept that must be considered.
Temperature
Similarly, the temperature in the tank is important, but it is not the key concept in analyzing the filling of an evacuated tank. The temperature of the fluid will change as it enters the tank, but this is a secondary effect that is influenced by the filling process rather than a key concept that must be considered.
Conclusion
In conclusion, the key concept in analyzing the filling of an evacuated tank is the enthalpy across the valve. This is because any changes in enthalpy can affect the temperature and pressure of the fluid, which can impact the performance and safety of the tank.
The values of heat and work transfer for the flow processes of a thermodynamic cycle are given below: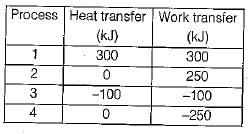 The thermal efficiency and work ratio for the cycle will be respectively
The thermal efficiency and work ratio for the cycle will be respectively- a)33% and 0.66
- b)66% and 0.36
- c)36% and 0.66
- d)33% and 0.36
Correct answer is option 'B'. Can you explain this answer?
The values of heat and work transfer for the flow processes of a thermodynamic cycle are given below:

The thermal efficiency and work ratio for the cycle will be respectively
a)
33% and 0.66
b)
66% and 0.36
c)
36% and 0.66
d)
33% and 0.36
|
|
Prateek Mukherjee answered |
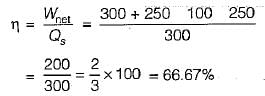
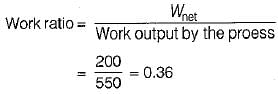
An ideal gas of mass m at state 1 expands to state 2 via three paths. If QA, QB and Qc represent the heat absorbed by the gas along three paths, then
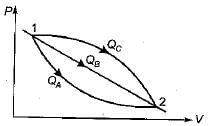
- a)QA < QB < QC
- b)QA > QB > QC
- c)QA < QB > QC
- d)QA > QB < QC
Correct answer is option 'A'. Can you explain this answer?
An ideal gas of mass m at state 1 expands to state 2 via three paths. If QA, QB and Qc represent the heat absorbed by the gas along three paths, then

a)
QA < QB < QC
b)
QA > QB > QC
c)
QA < QB > QC
d)
QA > QB < QC

|
Anuj Chakraborty answered |
from the first law of thermodynamics Q = U + W
here internal energy will be same for all the path,hence the required equation will be Q = W
now the path which have higher work done will also have higher heat .
so, W1<W2<W3
also,Q1<Q2<Q3
A key concept in analyzing the filling of an evacuated tank is- a)The mass flow rate into the tank remains constant.
- b)The enthalpy across a valve remains constant.
- c)The internal energy in the tank remains constant.
- d)The temperature in the tank remains constant
Correct answer is option 'B'. Can you explain this answer?
A key concept in analyzing the filling of an evacuated tank is
a)
The mass flow rate into the tank remains constant.
b)
The enthalpy across a valve remains constant.
c)
The internal energy in the tank remains constant.
d)
The temperature in the tank remains constant

|
Mihir Kulkarni answered |
The concept of analyzing the filling of an evacuated tank:
When analyzing the filling of an evacuated tank, there are several key concepts to consider. One of these key concepts is the enthalpy across a valve remaining constant.
Enthalpy:
Enthalpy is a thermodynamic property that describes the total energy of a system, including its internal energy and the work done on or by the system. In the context of a tank filling process, enthalpy is important because it accounts for both the internal energy of the fluid and the energy associated with the flow itself.
The significance of enthalpy remaining constant:
In the context of the filling of an evacuated tank, the enthalpy across a valve remaining constant is a key concept because it implies that there is no heat transfer or work done on the fluid during the filling process. This assumption is often made in idealized models of fluid flow, where the focus is on the mass flow rate and pressure changes.
Explanation of the other options:
a) The mass flow rate into the tank remains constant: While the mass flow rate may be constant during certain filling processes, it is not a key concept when analyzing the filling of an evacuated tank. The focus is more on the energy changes and pressure variations.
c) The internal energy in the tank remains constant: The internal energy of the fluid in the tank will change during the filling process as it gains energy from the surroundings. Therefore, this option is not correct.
d) The temperature in the tank remains constant: The temperature in the tank will generally increase during the filling process as the fluid gains energy. Therefore, this option is not correct.
Conclusion:
In conclusion, the key concept in analyzing the filling of an evacuated tank is that the enthalpy across a valve remains constant. This concept assumes that there is no heat transfer or work done on the fluid during the filling process. By considering this concept, engineers can better understand and model the behavior of fluid flow in tanks.
When analyzing the filling of an evacuated tank, there are several key concepts to consider. One of these key concepts is the enthalpy across a valve remaining constant.
Enthalpy:
Enthalpy is a thermodynamic property that describes the total energy of a system, including its internal energy and the work done on or by the system. In the context of a tank filling process, enthalpy is important because it accounts for both the internal energy of the fluid and the energy associated with the flow itself.
The significance of enthalpy remaining constant:
In the context of the filling of an evacuated tank, the enthalpy across a valve remaining constant is a key concept because it implies that there is no heat transfer or work done on the fluid during the filling process. This assumption is often made in idealized models of fluid flow, where the focus is on the mass flow rate and pressure changes.
Explanation of the other options:
a) The mass flow rate into the tank remains constant: While the mass flow rate may be constant during certain filling processes, it is not a key concept when analyzing the filling of an evacuated tank. The focus is more on the energy changes and pressure variations.
c) The internal energy in the tank remains constant: The internal energy of the fluid in the tank will change during the filling process as it gains energy from the surroundings. Therefore, this option is not correct.
d) The temperature in the tank remains constant: The temperature in the tank will generally increase during the filling process as the fluid gains energy. Therefore, this option is not correct.
Conclusion:
In conclusion, the key concept in analyzing the filling of an evacuated tank is that the enthalpy across a valve remains constant. This concept assumes that there is no heat transfer or work done on the fluid during the filling process. By considering this concept, engineers can better understand and model the behavior of fluid flow in tanks.
Chapter doubts & questions for First Law of Thermodynamics - Thermodynamics 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of First Law of Thermodynamics - Thermodynamics in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Thermodynamics
29 videos|150 docs|36 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup