Class 12 Exam > Class 12 Questions > P2O5 is heated with water to give [1991]a)Hyp...
Start Learning for Free
P2O5 is heated with water to give [1991]
- a)Hypophosphorous acid
- b)Phosphorous acid
- c)Hypophosphoric acid
- d)Orthophosphoric acid
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosph...
P2O5 have great affinity for water. The final product is orthophosphoric acid.
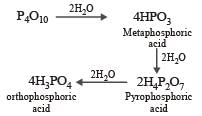
Most Upvoted Answer
P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosph...
Heating P2O5 with water results in the formation of orthophosphoric acid (H3PO4). Let's break down the process step by step:
1. P2O5 is a molecular compound known as diphosphorus pentoxide. It is a white, crystalline solid that is highly hygroscopic, meaning it readily absorbs water from the surrounding environment.
2. When P2O5 is exposed to water (H2O), it undergoes a hydrolysis reaction. Hydrolysis is a chemical reaction in which a compound reacts with water to produce new compounds.
3. In the case of P2O5, the hydrolysis reaction can be represented as follows:
P2O5 + 3H2O → 2H3PO4
4. The reaction shows that P2O5 reacts with three molecules of water to form two molecules of orthophosphoric acid (H3PO4). The balanced chemical equation indicates the stoichiometric ratio between P2O5 and H3PO4.
5. Orthophosphoric acid, also known as phosphoric acid, is a weak acid with the chemical formula H3PO4. It is a colorless, viscous liquid that is commonly used in various industries, including food and beverages, fertilizers, and detergents.
Overall, the reaction between P2O5 and water results in the formation of orthophosphoric acid (H3PO4). This reaction is an example of a hydrolysis reaction, where the compound P2O5 reacts with water to produce a different compound. It is important to note that the reaction requires three molecules of water for every molecule of P2O5 to form two molecules of orthophosphoric acid.
1. P2O5 is a molecular compound known as diphosphorus pentoxide. It is a white, crystalline solid that is highly hygroscopic, meaning it readily absorbs water from the surrounding environment.
2. When P2O5 is exposed to water (H2O), it undergoes a hydrolysis reaction. Hydrolysis is a chemical reaction in which a compound reacts with water to produce new compounds.
3. In the case of P2O5, the hydrolysis reaction can be represented as follows:
P2O5 + 3H2O → 2H3PO4
4. The reaction shows that P2O5 reacts with three molecules of water to form two molecules of orthophosphoric acid (H3PO4). The balanced chemical equation indicates the stoichiometric ratio between P2O5 and H3PO4.
5. Orthophosphoric acid, also known as phosphoric acid, is a weak acid with the chemical formula H3PO4. It is a colorless, viscous liquid that is commonly used in various industries, including food and beverages, fertilizers, and detergents.
Overall, the reaction between P2O5 and water results in the formation of orthophosphoric acid (H3PO4). This reaction is an example of a hydrolysis reaction, where the compound P2O5 reacts with water to produce a different compound. It is important to note that the reaction requires three molecules of water for every molecule of P2O5 to form two molecules of orthophosphoric acid.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer?
Question Description
P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer?.
P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer?.
Solutions for P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice P2O5 is heated with water to give [1991]a)Hypophosphorous acidb)Phosphorous acidc)Hypophosphoric acidd)Orthophosphoric acidCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















