Class 11 Exam > Class 11 Questions > When hydrochloric acid gas is treated with pr...
Start Learning for Free
When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]
- a)2-Chloropropane
- b)Allyl chloride
- c)No reaction
- d)n-Propyl chloride.
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
When hydrochloric acid gas is treated with propene in presence of benz...
Peroxide effect is observed only in case of HBr. Therefore, addition of HCl to propene even in the presence of benzyoyl peroxide occurs according to Markovnikov’s rule :
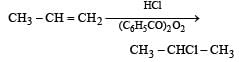
Most Upvoted Answer
When hydrochloric acid gas is treated with propene in presence of benz...
Reaction:
The reaction between hydrochloric acid gas and propene in the presence of benzoyl peroxide is an example of an electrophilic addition reaction.
Electrophilic Addition Reaction:
An electrophilic addition reaction involves the addition of an electrophile to a molecule, resulting in the formation of a new compound. In this case, the electrophile is the positively charged hydrogen atom in hydrochloric acid.
Benzoyl Peroxide:
Benzoyl peroxide is a radical initiator that is commonly used in organic chemistry reactions. It provides free radicals that initiate the reaction by abstracting a hydrogen atom from a reactant.
Propene:
Propene is an unsaturated hydrocarbon with a double bond between two of its carbon atoms. It is an alkene and can undergo addition reactions.
Step 1: Initiation
Benzoyl peroxide undergoes homolytic cleavage to form benzoyl radicals. Each benzoyl radical then abstracts a hydrogen atom from hydrochloric acid gas, resulting in the formation of a chlorine radical and a benzoyl chloride radical.
Step 2: Propagation
The chlorine radical reacts with propene by abstracting a hydrogen atom from one of its carbon atoms. This generates a propene radical, which is a highly reactive species.
Step 3: Termination
The propene radical can react with the chlorine radical to form 2-chloropropane. This is the desired product in this reaction.
Explanation of Correct Answer:
The correct answer is option 'A' - 2-chloropropane. This is because the reaction between hydrochloric acid gas and propene in the presence of benzoyl peroxide leads to the formation of 2-chloropropane as the main product.
The reaction proceeds through a radical mechanism, where benzoyl peroxide acts as a radical initiator. The benzoyl peroxide undergoes homolytic cleavage to form benzoyl radicals, which then abstract a hydrogen atom from hydrochloric acid gas. This results in the formation of a chlorine radical and a benzoyl chloride radical.
The chlorine radical then reacts with propene, abstracting a hydrogen atom from one of its carbon atoms. This generates a propene radical, which is highly reactive. The propene radical can further react with the chlorine radical, leading to the formation of 2-chloropropane.
Therefore, the correct product formed in this reaction is 2-chloropropane, which corresponds to option 'A'.
The reaction between hydrochloric acid gas and propene in the presence of benzoyl peroxide is an example of an electrophilic addition reaction.
Electrophilic Addition Reaction:
An electrophilic addition reaction involves the addition of an electrophile to a molecule, resulting in the formation of a new compound. In this case, the electrophile is the positively charged hydrogen atom in hydrochloric acid.
Benzoyl Peroxide:
Benzoyl peroxide is a radical initiator that is commonly used in organic chemistry reactions. It provides free radicals that initiate the reaction by abstracting a hydrogen atom from a reactant.
Propene:
Propene is an unsaturated hydrocarbon with a double bond between two of its carbon atoms. It is an alkene and can undergo addition reactions.
Step 1: Initiation
Benzoyl peroxide undergoes homolytic cleavage to form benzoyl radicals. Each benzoyl radical then abstracts a hydrogen atom from hydrochloric acid gas, resulting in the formation of a chlorine radical and a benzoyl chloride radical.
Step 2: Propagation
The chlorine radical reacts with propene by abstracting a hydrogen atom from one of its carbon atoms. This generates a propene radical, which is a highly reactive species.
Step 3: Termination
The propene radical can react with the chlorine radical to form 2-chloropropane. This is the desired product in this reaction.
Explanation of Correct Answer:
The correct answer is option 'A' - 2-chloropropane. This is because the reaction between hydrochloric acid gas and propene in the presence of benzoyl peroxide leads to the formation of 2-chloropropane as the main product.
The reaction proceeds through a radical mechanism, where benzoyl peroxide acts as a radical initiator. The benzoyl peroxide undergoes homolytic cleavage to form benzoyl radicals, which then abstract a hydrogen atom from hydrochloric acid gas. This results in the formation of a chlorine radical and a benzoyl chloride radical.
The chlorine radical then reacts with propene, abstracting a hydrogen atom from one of its carbon atoms. This generates a propene radical, which is highly reactive. The propene radical can further react with the chlorine radical, leading to the formation of 2-chloropropane.
Therefore, the correct product formed in this reaction is 2-chloropropane, which corresponds to option 'A'.

|
Explore Courses for Class 11 exam
|

|
Question Description
When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer?.
When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer?.
Solutions for When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer?, a detailed solution for When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]a)2-Chloropropaneb)Allyl chloridec)No reactiond)n-Propyl chloride.Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















