Class 12 Exam > Class 12 Questions > Propene, CH3CH = CH2 can be converted into 1-...
Start Learning for Free
Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]
- a)KMnO4 (alkaline)
- b)Osmium tetraoxide (OsO4/CH2Cl2)
- c)B6H6 and alk. H2O2
- d)O3/Zn
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. In...
KMnO4 (alkaline) and OsO4/CH2Cl2 are used for hydroxylation of double bond while O3/Zn is used for ozonolysis. Therefore, the right option is (c), i.e.,
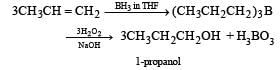
Most Upvoted Answer
Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. In...
Conversion of Propene to 1-Propanol:
Reagents:
- B6H6 (borane) and alk. H2O2
Explanation:
- Borane (B6H6) is used in hydroboration-oxidation reactions to convert alkenes into alcohols.
- In the presence of alk. H2O2, the borane adds across the double bond of propene to form a trialkylborane intermediate.
- The trialkylborane is then oxidized by alk. H2O2 to give the corresponding alcohol, which in this case is 1-propanol.
- This reaction is regioselective, leading to the formation of 1-propanol as the major product.
Other Options:
- KMnO4 (alkaline): This reagent is more suitable for the oxidation of primary alcohols or aldehydes to carboxylic acids, not for the conversion of propene to 1-propanol.
- Osmium tetraoxide (OsO4/CH2Cl2): This reagent is used for the dihydroxylation of alkenes, not for the specific conversion of propene to 1-propanol.
- O3/Zn: This reagent is used for the oxidative cleavage of alkenes to form carbonyl compounds, not for the conversion of propene to 1-propanol.
Therefore, the ideal set of reagents for converting propene into 1-propanol is B6H6 and alk. H2O2.
Reagents:
- B6H6 (borane) and alk. H2O2
Explanation:
- Borane (B6H6) is used in hydroboration-oxidation reactions to convert alkenes into alcohols.
- In the presence of alk. H2O2, the borane adds across the double bond of propene to form a trialkylborane intermediate.
- The trialkylborane is then oxidized by alk. H2O2 to give the corresponding alcohol, which in this case is 1-propanol.
- This reaction is regioselective, leading to the formation of 1-propanol as the major product.
Other Options:
- KMnO4 (alkaline): This reagent is more suitable for the oxidation of primary alcohols or aldehydes to carboxylic acids, not for the conversion of propene to 1-propanol.
- Osmium tetraoxide (OsO4/CH2Cl2): This reagent is used for the dihydroxylation of alkenes, not for the specific conversion of propene to 1-propanol.
- O3/Zn: This reagent is used for the oxidative cleavage of alkenes to form carbonyl compounds, not for the conversion of propene to 1-propanol.
Therefore, the ideal set of reagents for converting propene into 1-propanol is B6H6 and alk. H2O2.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer?
Question Description
Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer?.
Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]a)KMnO4 (alkaline)b)Osmium tetraoxide (OsO4/CH2Cl2)c)B6H6 and alk. H2O2d)O3/ZnCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















