Class 11 Exam > Class 11 Questions > In preparation of alkene from alcohol using A...
Start Learning for Free
In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]
- a)Temperature
- b)Concentration
- c)Surface area of Al2O3
- d)Porosity of Al2O3
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
In preparation of alkene from alcohol using Al2O3 which is effective f...
Temperature
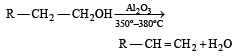
While at 220º – 250ºC it forms ether
Most Upvoted Answer
In preparation of alkene from alcohol using Al2O3 which is effective f...
Effective Factor in the Preparation of Alkene from Alcohol using Al2O3: Temperature
When preparing alkene from alcohol using Al2O3 as a catalyst, the most effective factor among the given options is temperature. Let's understand why temperature is the key factor in this process.
Role of Al2O3 Catalyst
- Al2O3 is commonly used as a catalyst in the dehydration of alcohols to form alkenes.
- The catalyst provides an alternative reaction pathway with lower activation energy, allowing the reaction to occur at a faster rate.
- It acts by adsorbing the alcohol molecules on its surface, facilitating the removal of water molecules and promoting the formation of the alkene.
Effect of Temperature on the Reaction
- Temperature affects the rate of reaction by influencing the kinetic energy of the reactant molecules.
- As temperature increases, the kinetic energy of the molecules also increases, resulting in more frequent and energetic collisions between the reactant molecules.
- The increased collision frequency and energy enhance the chances of successful collision, leading to a higher reaction rate.
- In the case of the dehydration of alcohol to alkene, higher temperatures provide the necessary energy to break the O-H bond in the alcohol molecule, allowing the formation of the alkene.
- Therefore, the reaction rate and yield of alkene increase with increasing temperature.
Optimum Temperature
- While higher temperatures favor the reaction rate, excessively high temperatures may lead to undesired side reactions or thermal decomposition of the reactants or products.
- The optimum temperature for the preparation of alkene from alcohol using Al2O3 catalyst depends on the specific alcohol and reaction conditions.
- It is essential to strike a balance between achieving a high reaction rate and maximizing the selectivity and yield of the desired alkene.
- The optimum temperature can be determined through experimentation and optimization of reaction conditions.
In conclusion, the temperature is the most effective factor in the preparation of alkene from alcohol using Al2O3 as a catalyst. It influences the reaction rate by providing the necessary energy for the breaking of O-H bonds in the alcohol molecule. However, it is important to carefully choose the temperature to achieve both a high reaction rate and a high yield of the desired alkene.
When preparing alkene from alcohol using Al2O3 as a catalyst, the most effective factor among the given options is temperature. Let's understand why temperature is the key factor in this process.
Role of Al2O3 Catalyst
- Al2O3 is commonly used as a catalyst in the dehydration of alcohols to form alkenes.
- The catalyst provides an alternative reaction pathway with lower activation energy, allowing the reaction to occur at a faster rate.
- It acts by adsorbing the alcohol molecules on its surface, facilitating the removal of water molecules and promoting the formation of the alkene.
Effect of Temperature on the Reaction
- Temperature affects the rate of reaction by influencing the kinetic energy of the reactant molecules.
- As temperature increases, the kinetic energy of the molecules also increases, resulting in more frequent and energetic collisions between the reactant molecules.
- The increased collision frequency and energy enhance the chances of successful collision, leading to a higher reaction rate.
- In the case of the dehydration of alcohol to alkene, higher temperatures provide the necessary energy to break the O-H bond in the alcohol molecule, allowing the formation of the alkene.
- Therefore, the reaction rate and yield of alkene increase with increasing temperature.
Optimum Temperature
- While higher temperatures favor the reaction rate, excessively high temperatures may lead to undesired side reactions or thermal decomposition of the reactants or products.
- The optimum temperature for the preparation of alkene from alcohol using Al2O3 catalyst depends on the specific alcohol and reaction conditions.
- It is essential to strike a balance between achieving a high reaction rate and maximizing the selectivity and yield of the desired alkene.
- The optimum temperature can be determined through experimentation and optimization of reaction conditions.
In conclusion, the temperature is the most effective factor in the preparation of alkene from alcohol using Al2O3 as a catalyst. It influences the reaction rate by providing the necessary energy for the breaking of O-H bonds in the alcohol molecule. However, it is important to carefully choose the temperature to achieve both a high reaction rate and a high yield of the desired alkene.

|
Explore Courses for Class 11 exam
|

|
Question Description
In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer?.
In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer?.
Solutions for In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer?, a detailed solution for In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]a)Temperatureb)Concentrationc)Surface area of Al2O3d)Porosity of Al2O3Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















