Chemistry Exam > Chemistry Questions > Arrange the following compound in order of th...
Start Learning for Free
Arrange the following compound in order of their acidity. (most to least)
(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOH
(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOH
- a)IV > III > II > V > I
- b)IV > III > II > I > V
- c)V > II > III > IV > I
- d)V > III > IV > II > I
Correct answer is option 'A'. Can you explain this answer?
Most Upvoted Answer
Arrange the following compound in order of their acidity. (most to lea...
The order of acidity can be determined by assessing the stability of the conjugate base. The more stable the conjugate base, the stronger the acid.
In this case, the conjugate bases are CH3CH2O-, CFH2CO2-, CF2HCO2-, and CF3COO-.
To assess the stability of the conjugate bases, we can consider the following factors:
1. Electronegativity: The more electronegative the atom carrying the negative charge, the more stable the conjugate base. In this case, the carbon atom is more electronegative than the hydrogen atom, and the fluorine atom is more electronegative than the carbon atom. Therefore, the order of electronegativity is: F > C > H.
2. Inductive effect: The more electron-withdrawing groups attached to the atom carrying the negative charge, the more stable the conjugate base. In this case, the CF3 group is more electron-withdrawing than the CF2H and CFH2 groups. Therefore, the order of the inductive effect is: CF3 > CF2H > CFH2.
Based on these factors, we can arrange the compounds in order of their acidity:
IV (CF3COOH): The CF3 group is highly electronegative and strongly electron-withdrawing, making the conjugate base CF3COO- highly stable.
V (CF3COOH): Similar to compound IV, the CF3 group is highly electronegative and strongly electron-withdrawing, making the conjugate base CF3COO- highly stable.
III (CF2HCO2H): The CF2H group is less electronegative than the CF3 group but still more electronegative than the hydrogen atom in compound I. It is also electron-withdrawing, making the conjugate base CF2HCO2- more stable than the conjugate base of compound I.
II (CFH2CO2H): The CFH2 group is less electronegative and less electron-withdrawing than the CF2H group, making the conjugate base CFH2CO2- less stable than the conjugate base of compound III.
I (CH3CH2OH): The CH3CH2O- conjugate base has no electronegative or electron-withdrawing groups attached to the carbon atom, making it the least stable among the given compounds.
Therefore, the correct order of acidity is: IV > V > III > II > I.
In this case, the conjugate bases are CH3CH2O-, CFH2CO2-, CF2HCO2-, and CF3COO-.
To assess the stability of the conjugate bases, we can consider the following factors:
1. Electronegativity: The more electronegative the atom carrying the negative charge, the more stable the conjugate base. In this case, the carbon atom is more electronegative than the hydrogen atom, and the fluorine atom is more electronegative than the carbon atom. Therefore, the order of electronegativity is: F > C > H.
2. Inductive effect: The more electron-withdrawing groups attached to the atom carrying the negative charge, the more stable the conjugate base. In this case, the CF3 group is more electron-withdrawing than the CF2H and CFH2 groups. Therefore, the order of the inductive effect is: CF3 > CF2H > CFH2.
Based on these factors, we can arrange the compounds in order of their acidity:
IV (CF3COOH): The CF3 group is highly electronegative and strongly electron-withdrawing, making the conjugate base CF3COO- highly stable.
V (CF3COOH): Similar to compound IV, the CF3 group is highly electronegative and strongly electron-withdrawing, making the conjugate base CF3COO- highly stable.
III (CF2HCO2H): The CF2H group is less electronegative than the CF3 group but still more electronegative than the hydrogen atom in compound I. It is also electron-withdrawing, making the conjugate base CF2HCO2- more stable than the conjugate base of compound I.
II (CFH2CO2H): The CFH2 group is less electronegative and less electron-withdrawing than the CF2H group, making the conjugate base CFH2CO2- less stable than the conjugate base of compound III.
I (CH3CH2OH): The CH3CH2O- conjugate base has no electronegative or electron-withdrawing groups attached to the carbon atom, making it the least stable among the given compounds.
Therefore, the correct order of acidity is: IV > V > III > II > I.
Community Answer
Arrange the following compound in order of their acidity. (most to lea...
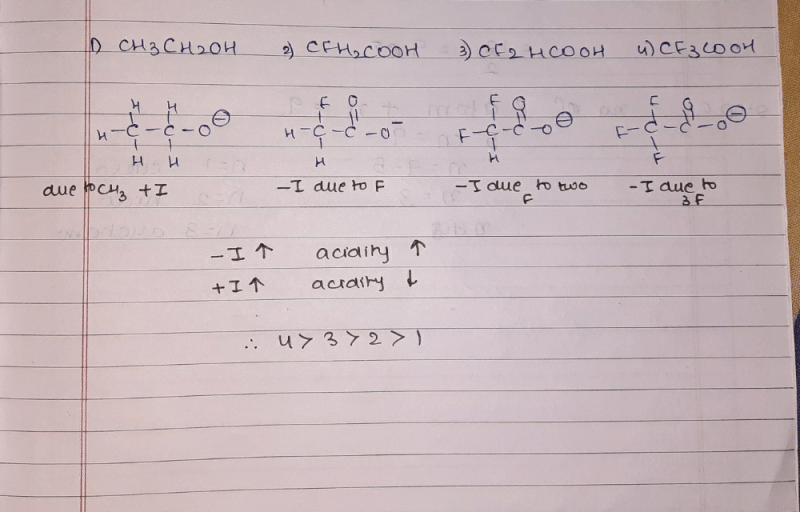

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer?
Question Description
Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer?.
Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer?.
Solutions for Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Arrange the following compound in order of their acidity. (most to least)(I) CH3CH2OH (II) CFH2CO2H (III) CF2HCO2H (IV) CF3COOH (V) CF3COOHa)IV > III > II > V > Ib)IV > III > II > I > Vc)V > II > III > IV > Id)V > III > IV > II > ICorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















