Class 12 Exam > Class 12 Questions > Comprehension TypeDirection (Q. Nos. 16and 17...
Start Learning for Free
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
When crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN- ions but give test for new ion [Fe(CN)6]4- called ferrocyanide ion.
Q.
The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion are
- a)sp3, tetrahedral, diamagnetic
- b)sp3, tetrahedral, paramagnetic
- c)dsp2, square planar, diamagnetic
- d)dsp2, square planar, paramagnetic
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a...
Cu(Z = 29) copper configuration (3d104s1)
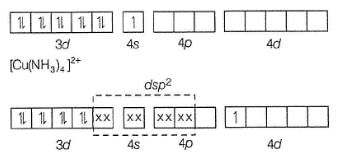
Hence, [Cu(NH3)4]2+ has square planar geometry and 1 unpaired electron in Ad orbital which is transferred from 3d orbital with magnetic moment 1.73 BM.

Hence, [Cu(NH3)4]2+ has square planar geometry and 1 unpaired electron in Ad orbital which is transferred from 3d orbital with magnetic moment 1.73 BM.
Most Upvoted Answer
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a...
Answer:
The hybridisation and geometry of [Cu(NH3)4]2 ion can be determined by considering the coordination number and the nature of ligands attached to the central metal ion.
Hybridisation:
In [Cu(NH3)4]2, the coordination number of copper (Cu) is 4, which suggests sp3 hybridisation. This means that the copper atom in the complex is surrounded by four electron pairs, including four ammonia (NH3) ligands.
Geometry:
The sp3 hybridisation corresponds to a tetrahedral geometry. In a tetrahedral arrangement, the four ammonia ligands are arranged around the copper ion in a three-dimensional manner, with bond angles of approximately 109.5 degrees.
Magnetic Property:
To determine the magnetic property of [Cu(NH3)4]2, we need to consider the electronic configuration and the presence of unpaired electrons.
Cu2+ ion has an electronic configuration of [Ar]3d9. In [Cu(NH3)4]2, four ammonia ligands donate their electron pairs to the copper ion, resulting in the formation of coordinate bonds. As a result, the copper ion gains four additional electrons, leading to the formation of a d10 configuration.
In a d10 configuration, all the d orbitals are completely filled, and there are no unpaired electrons. Therefore, [Cu(NH3)4]2 is diamagnetic. Diamagnetic substances are those that do not contain any unpaired electrons and are not attracted to a magnetic field.
Summary:
- Hybridisation: sp3
- Geometry: Tetrahedral
- Magnetic Property: Diamagnetic
Overall, the [Cu(NH3)4]2 ion has sp3 hybridisation, a tetrahedral geometry, and is diamagnetic.
The hybridisation and geometry of [Cu(NH3)4]2 ion can be determined by considering the coordination number and the nature of ligands attached to the central metal ion.
Hybridisation:
In [Cu(NH3)4]2, the coordination number of copper (Cu) is 4, which suggests sp3 hybridisation. This means that the copper atom in the complex is surrounded by four electron pairs, including four ammonia (NH3) ligands.
Geometry:
The sp3 hybridisation corresponds to a tetrahedral geometry. In a tetrahedral arrangement, the four ammonia ligands are arranged around the copper ion in a three-dimensional manner, with bond angles of approximately 109.5 degrees.
Magnetic Property:
To determine the magnetic property of [Cu(NH3)4]2, we need to consider the electronic configuration and the presence of unpaired electrons.
Cu2+ ion has an electronic configuration of [Ar]3d9. In [Cu(NH3)4]2, four ammonia ligands donate their electron pairs to the copper ion, resulting in the formation of coordinate bonds. As a result, the copper ion gains four additional electrons, leading to the formation of a d10 configuration.
In a d10 configuration, all the d orbitals are completely filled, and there are no unpaired electrons. Therefore, [Cu(NH3)4]2 is diamagnetic. Diamagnetic substances are those that do not contain any unpaired electrons and are not attracted to a magnetic field.
Summary:
- Hybridisation: sp3
- Geometry: Tetrahedral
- Magnetic Property: Diamagnetic
Overall, the [Cu(NH3)4]2 ion has sp3 hybridisation, a tetrahedral geometry, and is diamagnetic.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Question Description
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer?.
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageWhen crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN-ions but give test for new ion [Fe(CN)6]4-called ferrocyanide ion.Q.The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion area)sp3, tetrahedral, diamagneticb)sp3, tetrahedral, paramagneticc)dsp2, square planar, diamagneticd)dsp2, square planar, paramagneticCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
















