Class 11 Exam > Class 11 Questions > Which of the following molecules are stabilis...
Start Learning for Free
Which of the following molecules are stabilised by intramolecular H-bonding?
- a)o-nitrophenol
- b)p-nitrophenol
- c)Acetyl acetone in enolic form
- d)Benzoic acid
Correct answer is option 'A,C'. Can you explain this answer?
Verified Answer
Which of the following molecules are stabilised by intramolecular H-bo...
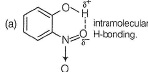
(a) intramolecular H-bonding.

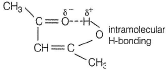
Most Upvoted Answer
Which of the following molecules are stabilised by intramolecular H-bo...
Stabilization by intramolecular hydrogen bonding occurs when a hydrogen atom is bonded to an electronegative atom (such as oxygen or nitrogen) and forms a hydrogen bond with another electronegative atom within the same molecule. This type of bonding can lead to unique structural and chemical properties of the molecule.
Let's analyze each option to determine if it exhibits intramolecular hydrogen bonding:
a) o-nitrophenol:
In o-nitrophenol, the hydrogen atom bonded to the hydroxyl group (-OH) can form a hydrogen bond with the oxygen atom of the nitro group (-NO2). This intramolecular hydrogen bonding stabilizes the molecule by forming a ring-like structure, enhancing its stability.
b) p-nitrophenol:
In p-nitrophenol, the hydrogen atom bonded to the hydroxyl group (-OH) does not have an electronegative atom nearby to form an intramolecular hydrogen bond. Therefore, this molecule does not exhibit intramolecular hydrogen bonding.
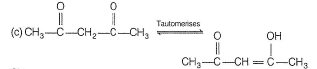
c) Acetyl acetone in enolic form:
In the enolic form of acetyl acetone, the hydrogen atom bonded to the hydroxyl group (-OH) can form an intramolecular hydrogen bond with the oxygen atom of the carbonyl group (-C=O). This hydrogen bond stabilizes the molecule and influences its tautomeric equilibrium.
d) Benzoic acid:
In benzoic acid, the hydrogen atom bonded to the carboxyl group (-COOH) can form an intramolecular hydrogen bond with one of the oxygen atoms of the carbonyl group (-C=O). This hydrogen bond stabilizes the molecule and influences its structure and reactivity.
Overall, the molecules that are stabilized by intramolecular hydrogen bonding are o-nitrophenol and acetyl acetone in enolic form (option A and option C). These hydrogen bonds play a crucial role in the stability, structure, and properties of these molecules.
Let's analyze each option to determine if it exhibits intramolecular hydrogen bonding:
a) o-nitrophenol:
In o-nitrophenol, the hydrogen atom bonded to the hydroxyl group (-OH) can form a hydrogen bond with the oxygen atom of the nitro group (-NO2). This intramolecular hydrogen bonding stabilizes the molecule by forming a ring-like structure, enhancing its stability.
b) p-nitrophenol:
In p-nitrophenol, the hydrogen atom bonded to the hydroxyl group (-OH) does not have an electronegative atom nearby to form an intramolecular hydrogen bond. Therefore, this molecule does not exhibit intramolecular hydrogen bonding.
c) Acetyl acetone in enolic form:
In the enolic form of acetyl acetone, the hydrogen atom bonded to the hydroxyl group (-OH) can form an intramolecular hydrogen bond with the oxygen atom of the carbonyl group (-C=O). This hydrogen bond stabilizes the molecule and influences its tautomeric equilibrium.
d) Benzoic acid:
In benzoic acid, the hydrogen atom bonded to the carboxyl group (-COOH) can form an intramolecular hydrogen bond with one of the oxygen atoms of the carbonyl group (-C=O). This hydrogen bond stabilizes the molecule and influences its structure and reactivity.
Overall, the molecules that are stabilized by intramolecular hydrogen bonding are o-nitrophenol and acetyl acetone in enolic form (option A and option C). These hydrogen bonds play a crucial role in the stability, structure, and properties of these molecules.
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following molecules are stabilised by intramolecular H-bo...
A

|
Explore Courses for Class 11 exam
|

|
Question Description
Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer?.
Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer?.
Solutions for Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer?, a detailed solution for Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer? has been provided alongside types of Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following molecules are stabilised by intramolecular H-bonding?a)o-nitrophenolb)p-nitrophenolc)Acetyl acetone in enolic formd)Benzoic acidCorrect answer is option 'A,C'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















