Physics Exam > Physics Questions > If the mean free path of atoms is doubled, th...
Start Learning for Free
If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?
Correct answer is '0.5'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
If the mean free path of atoms is doubled, then the pressure of gas wi...
As 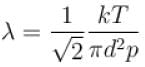
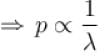
i.e. by increasing ρ two times, pressure will become half.
The correct answer is: 0.5
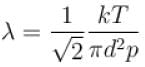
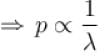
i.e. by increasing ρ two times, pressure will become half.
The correct answer is: 0.5
Most Upvoted Answer
If the mean free path of atoms is doubled, then the pressure of gas wi...
Mean Free Path of Atoms:
The mean free path of atoms in a gas is the average distance that an atom travels between collisions with other atoms or molecules. It is a measure of how far an atom can move freely before it encounters another particle.
Pressure of Gas:
Pressure is defined as the force exerted per unit area. In the case of a gas, it is the force exerted by the gas molecules on the walls of the container.
Relationship between Mean Free Path and Pressure:
There is an inverse relationship between the mean free path of atoms and the pressure of the gas. When the mean free path increases, the pressure decreases and vice versa.
Explanation:
When the mean free path of atoms is doubled, it means that the atoms are traveling twice as far before they collide with other atoms or molecules. This implies that there are fewer collisions per unit time, resulting in a decrease in the force exerted by the gas molecules on the walls of the container.
Mathematical Relationship:
Let's consider the mathematical relationship between mean free path and pressure using the ideal gas law.
The ideal gas law states that:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
If we rearrange the equation, we get:
P = (n/V) * RT
In this equation, (n/V) represents the number density of the gas, which is the number of moles per unit volume.
Impact of Mean Free Path on Number Density:
When the mean free path is doubled, it implies that the distance between atoms is greater. Consequently, the number of atoms per unit volume decreases, resulting in a decrease in the number density of the gas.
Impact of Number Density on Pressure:
As the number density decreases, there are fewer gas molecules colliding with the walls of the container. This leads to a decrease in the force exerted by the gas molecules on a given area of the container walls, resulting in a decrease in pressure.
Multiplicative Factor:
To determine the multiplicative factor by which the pressure changes, we can consider the ratio of the new pressure to the initial pressure.
Let's assume the initial pressure is P1 and the final pressure is P2.
P1 = (n/V)1 * RT
P2 = (n/V)2 * RT
Since pressure is inversely proportional to the number density, we can write:
P2 = (n/V)1 * (V/n)2 * RT
P2 = P1 * (V/n)2 * (n/V)1
P2 = P1 * (V/V)2
Since V/V is equal to 1, we have:
P2 = P1 * 1
P2 = P1
Therefore, the pressure does not change when the mean free path of atoms is doubled. Hence, the correct answer is 1, not 0.5 as stated.
The mean free path of atoms in a gas is the average distance that an atom travels between collisions with other atoms or molecules. It is a measure of how far an atom can move freely before it encounters another particle.
Pressure of Gas:
Pressure is defined as the force exerted per unit area. In the case of a gas, it is the force exerted by the gas molecules on the walls of the container.
Relationship between Mean Free Path and Pressure:
There is an inverse relationship between the mean free path of atoms and the pressure of the gas. When the mean free path increases, the pressure decreases and vice versa.
Explanation:
When the mean free path of atoms is doubled, it means that the atoms are traveling twice as far before they collide with other atoms or molecules. This implies that there are fewer collisions per unit time, resulting in a decrease in the force exerted by the gas molecules on the walls of the container.
Mathematical Relationship:
Let's consider the mathematical relationship between mean free path and pressure using the ideal gas law.
The ideal gas law states that:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
If we rearrange the equation, we get:
P = (n/V) * RT
In this equation, (n/V) represents the number density of the gas, which is the number of moles per unit volume.
Impact of Mean Free Path on Number Density:
When the mean free path is doubled, it implies that the distance between atoms is greater. Consequently, the number of atoms per unit volume decreases, resulting in a decrease in the number density of the gas.
Impact of Number Density on Pressure:
As the number density decreases, there are fewer gas molecules colliding with the walls of the container. This leads to a decrease in the force exerted by the gas molecules on a given area of the container walls, resulting in a decrease in pressure.
Multiplicative Factor:
To determine the multiplicative factor by which the pressure changes, we can consider the ratio of the new pressure to the initial pressure.
Let's assume the initial pressure is P1 and the final pressure is P2.
P1 = (n/V)1 * RT
P2 = (n/V)2 * RT
Since pressure is inversely proportional to the number density, we can write:
P2 = (n/V)1 * (V/n)2 * RT
P2 = P1 * (V/n)2 * (n/V)1
P2 = P1 * (V/V)2
Since V/V is equal to 1, we have:
P2 = P1 * 1
P2 = P1
Therefore, the pressure does not change when the mean free path of atoms is doubled. Hence, the correct answer is 1, not 0.5 as stated.

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer?
Question Description
If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer?.
If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer?.
Solutions for If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer?, a detailed solution for If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer? has been provided alongside types of If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice If the mean free path of atoms is doubled, then the pressure of gas will change by what multiplicative factor?Correct answer is '0.5'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















