Class 9 Exam > Class 9 Questions > Difference between osmosis and diffusion
Start Learning for Free
Difference between osmosis and diffusion
Verified Answer
Difference between osmosis and diffusion
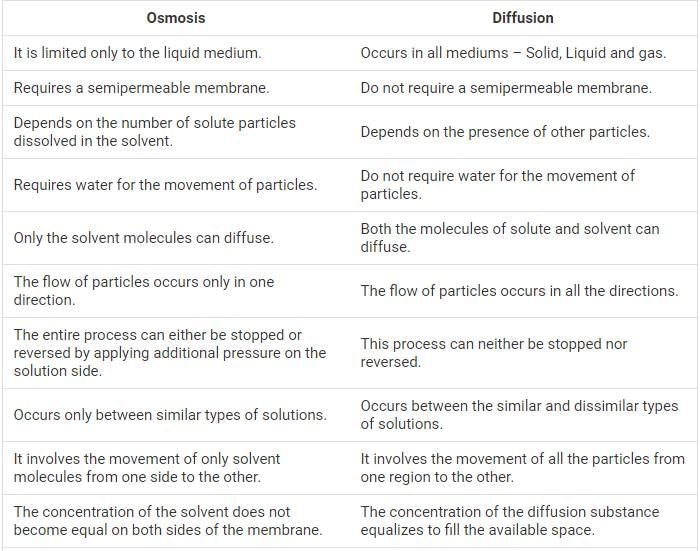
 This question is part of UPSC exam. View all Class 9 courses
This question is part of UPSC exam. View all Class 9 courses
Most Upvoted Answer
Difference between osmosis and diffusion
Introduction:
Osmosis and diffusion are both fundamental processes that occur in various biological and chemical systems. While both processes involve the movement of molecules from an area of high concentration to an area of low concentration, they differ in several key aspects. This response will provide a detailed explanation of the differences between osmosis and diffusion.
Osmosis:
Osmosis is a specific type of diffusion that involves the movement of water molecules across a semi-permeable membrane. It occurs when there is a concentration gradient of solute molecules on either side of the membrane, resulting in the movement of water to equalize the concentrations. Osmosis can be understood through the following key points:
1. Semi-permeable membrane: Osmosis occurs only when there is a semi-permeable membrane present, which allows the passage of water molecules but restricts the movement of solute molecules.
2. Water movement: In osmosis, water molecules move from an area of lower solute concentration (hypotonic solution) to an area of higher solute concentration (hypertonic solution) until equilibrium is reached.
3. Direction of movement: Osmosis is a passive process, meaning it does not require energy input. The direction of water movement is determined by the relative concentrations of solute molecules on either side of the membrane.
Diffusion:
Diffusion, on the other hand, refers to the movement of molecules or particles from an area of higher concentration to an area of lower concentration. It occurs in various environments and does not require a semi-permeable membrane. The following points help understand diffusion:
1. Concentration gradient: Diffusion occurs due to the presence of a concentration gradient. Molecules move randomly and collide with each other, leading to their dispersion.
2. Random movement: In diffusion, molecules move in a random fashion, driven by their own kinetic energy. This movement continues until the concentration becomes evenly distributed.
3. No membrane requirement: Unlike osmosis, diffusion can occur in any medium, including gases, liquids, and solids. It does not necessitate the presence of a semi-permeable membrane.
Conclusion:
In summary, osmosis is a specific type of diffusion that involves the movement of water across a semi-permeable membrane in response to a solute concentration gradient. Osmosis is driven by the need to equalize the solute concentrations on either side of the membrane. On the other hand, diffusion refers to the movement of molecules or particles from an area of higher concentration to an area of lower concentration, occurring in the absence of a membrane. Both osmosis and diffusion are important processes that facilitate the movement of substances in biological and chemical systems.
Osmosis and diffusion are both fundamental processes that occur in various biological and chemical systems. While both processes involve the movement of molecules from an area of high concentration to an area of low concentration, they differ in several key aspects. This response will provide a detailed explanation of the differences between osmosis and diffusion.
Osmosis:
Osmosis is a specific type of diffusion that involves the movement of water molecules across a semi-permeable membrane. It occurs when there is a concentration gradient of solute molecules on either side of the membrane, resulting in the movement of water to equalize the concentrations. Osmosis can be understood through the following key points:
1. Semi-permeable membrane: Osmosis occurs only when there is a semi-permeable membrane present, which allows the passage of water molecules but restricts the movement of solute molecules.
2. Water movement: In osmosis, water molecules move from an area of lower solute concentration (hypotonic solution) to an area of higher solute concentration (hypertonic solution) until equilibrium is reached.
3. Direction of movement: Osmosis is a passive process, meaning it does not require energy input. The direction of water movement is determined by the relative concentrations of solute molecules on either side of the membrane.
Diffusion:
Diffusion, on the other hand, refers to the movement of molecules or particles from an area of higher concentration to an area of lower concentration. It occurs in various environments and does not require a semi-permeable membrane. The following points help understand diffusion:
1. Concentration gradient: Diffusion occurs due to the presence of a concentration gradient. Molecules move randomly and collide with each other, leading to their dispersion.
2. Random movement: In diffusion, molecules move in a random fashion, driven by their own kinetic energy. This movement continues until the concentration becomes evenly distributed.
3. No membrane requirement: Unlike osmosis, diffusion can occur in any medium, including gases, liquids, and solids. It does not necessitate the presence of a semi-permeable membrane.
Conclusion:
In summary, osmosis is a specific type of diffusion that involves the movement of water across a semi-permeable membrane in response to a solute concentration gradient. Osmosis is driven by the need to equalize the solute concentrations on either side of the membrane. On the other hand, diffusion refers to the movement of molecules or particles from an area of higher concentration to an area of lower concentration, occurring in the absence of a membrane. Both osmosis and diffusion are important processes that facilitate the movement of substances in biological and chemical systems.
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
Difference between osmosis and diffusion
Question Description
Difference between osmosis and diffusion for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Difference between osmosis and diffusion covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Difference between osmosis and diffusion.
Difference between osmosis and diffusion for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Difference between osmosis and diffusion covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Difference between osmosis and diffusion.
Solutions for Difference between osmosis and diffusion in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of Difference between osmosis and diffusion defined & explained in the simplest way possible. Besides giving the explanation of
Difference between osmosis and diffusion, a detailed solution for Difference between osmosis and diffusion has been provided alongside types of Difference between osmosis and diffusion theory, EduRev gives you an
ample number of questions to practice Difference between osmosis and diffusion tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























