Physics Exam > Physics Questions > Five identifiable particles are distributed i...
Start Learning for Free
Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?
Select one or more:
Select one or more:
- a)N1 = 2
- b)N3 = 1
- c)N1 = 3
- d)N2 = 1
Correct answer is option 'B,C,D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Five identifiable particles are distributed in three non-degenerate le...
As the levels are non-degenerate, there is only one state for each energy.
Let the number of particles occupying the 3 energy states be N1, N2, and N3 respectively
where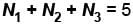
The particles are identifiable, the number of ways of choosing the particles is
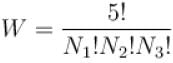
The energy of system is
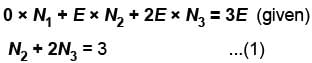
Now, the most probable distribution is the one in which W is a maximum, subject to constraint given by equation (1)
Thus if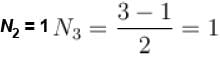
and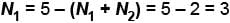
If N2 = 3, N3 = 0
and N1 = 5 – (3 + 0) = 2
No other distribution are possible
For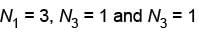
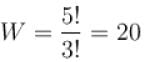
So must probable distribution is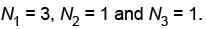
The correct answers are: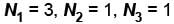
Let the number of particles occupying the 3 energy states be N1, N2, and N3 respectively
where
The particles are identifiable, the number of ways of choosing the particles is
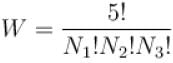
The energy of system is
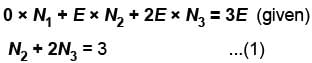
Now, the most probable distribution is the one in which W is a maximum, subject to constraint given by equation (1)
Thus if
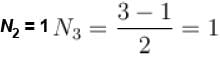
and
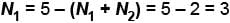
If N2 = 3, N3 = 0
and N1 = 5 – (3 + 0) = 2
No other distribution are possible
For
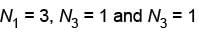
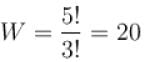
So must probable distribution is
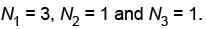
The correct answers are:
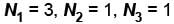
Most Upvoted Answer
Five identifiable particles are distributed in three non-degenerate le...
Most Probable Distribution of Particles
Given:
- Five identifiable particles
- Three non-degenerate energy levels: 0, E, and 2E
- Total energy of 3E
To determine the most probable distribution of particles, we need to find the combination of options that satisfies the given conditions.
1. Determine the number of particles in each energy level:
- Let N1 be the number of particles in the 0 energy level.
- Let N2 be the number of particles in the E energy level.
- Let N3 be the number of particles in the 2E energy level.
2. From the given total energy of 3E, we can write the equation for the total energy:
0*N1 + E*N2 + 2E*N3 = 3E
Simplifying the equation, we have:
E*N2 + 2E*N3 = 3E
Dividing both sides by E, we get:
N2 + 2N3 = 3
3. Possible combinations of options:
a) N1 = 2
b) N3 = 1
c) N1 = 3
d) N2 = 1
Let's analyze each option and check if they satisfy the equation.
Option a) N1 = 2:
If N1 = 2, then N2 + 2N3 = 3 becomes N2 + 2N3 = 1.
This equation cannot be satisfied since N2 and N3 cannot take fractional values. Therefore, option a) is not valid.
Option b) N3 = 1:
If N3 = 1, then N2 + 2N3 = 3 becomes N2 + 2 = 3.
Simplifying, we have N2 = 1.
This option satisfies the equation, so option b) is valid.
Option c) N1 = 3:
If N1 = 3, then N2 + 2N3 = 3 becomes N2 + 2N3 = 0.
This equation cannot be satisfied since N2 and N3 cannot take negative values. Therefore, option c) is not valid.
Option d) N2 = 1:
If N2 = 1, then N2 + 2N3 = 3 becomes 1 + 2N3 = 3.
Simplifying, we have N3 = 1.
This option satisfies the equation, so option d) is valid.
Therefore, the most probable distributions for a total energy of 3E correspond to options b), c), and d).
Given:
- Five identifiable particles
- Three non-degenerate energy levels: 0, E, and 2E
- Total energy of 3E
To determine the most probable distribution of particles, we need to find the combination of options that satisfies the given conditions.
1. Determine the number of particles in each energy level:
- Let N1 be the number of particles in the 0 energy level.
- Let N2 be the number of particles in the E energy level.
- Let N3 be the number of particles in the 2E energy level.
2. From the given total energy of 3E, we can write the equation for the total energy:
0*N1 + E*N2 + 2E*N3 = 3E
Simplifying the equation, we have:
E*N2 + 2E*N3 = 3E
Dividing both sides by E, we get:
N2 + 2N3 = 3
3. Possible combinations of options:
a) N1 = 2
b) N3 = 1
c) N1 = 3
d) N2 = 1
Let's analyze each option and check if they satisfy the equation.
Option a) N1 = 2:
If N1 = 2, then N2 + 2N3 = 3 becomes N2 + 2N3 = 1.
This equation cannot be satisfied since N2 and N3 cannot take fractional values. Therefore, option a) is not valid.
Option b) N3 = 1:
If N3 = 1, then N2 + 2N3 = 3 becomes N2 + 2 = 3.
Simplifying, we have N2 = 1.
This option satisfies the equation, so option b) is valid.
Option c) N1 = 3:
If N1 = 3, then N2 + 2N3 = 3 becomes N2 + 2N3 = 0.
This equation cannot be satisfied since N2 and N3 cannot take negative values. Therefore, option c) is not valid.
Option d) N2 = 1:
If N2 = 1, then N2 + 2N3 = 3 becomes 1 + 2N3 = 3.
Simplifying, we have N3 = 1.
This option satisfies the equation, so option d) is valid.
Therefore, the most probable distributions for a total energy of 3E correspond to options b), c), and d).

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer?
Question Description
Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer?.
Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer?.
Solutions for Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer?, a detailed solution for Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer? has been provided alongside types of Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Five identifiable particles are distributed in three non-degenerate levels with energies 0, E and 2E. The most probable distributions for total energy, 3E corresponds to what combination of options given below?Select one or more:a)N1 = 2b)N3 = 1c)N1 = 3d)N2 = 1Correct answer is option 'B,C,D'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















