Physics Exam > Physics Questions > A system of N non-interacting and distinguish...
Start Learning for Free
A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?
Correct answer is '3'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
A system of N non-interacting and distinguishable particles of spin1 i...
Number of microstates Ω = 3N
Entropy is given by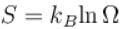
∴ S = NKBln 3
⇒ x = 3
The correct answer is: 3
Entropy is given by
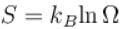
∴ S = NKBln 3
⇒ x = 3
The correct answer is: 3
Most Upvoted Answer
A system of N non-interacting and distinguishable particles of spin1 i...
The spin of a particle is a quantum mechanical property that can take on discrete values. In this case, the particles have a spin of 1, which means that their spin can take on three possible values: +1, 0, or -1.
To determine the entropy of the system, we need to calculate the number of microstates, Ω, that correspond to a given macrostate. The macrostate is defined by the total spin of the system, which can range from -N to +N in steps of 2.
The number of microstates for each macrostate can be determined using the formula for the degeneracy of a system with multiple particles and multiple energy levels. In this case, the energy levels correspond to the possible spin values of the particles.
The degeneracy is given by the formula:
Ω = (2J + 1)^(N_up) * (2J + 1)^(N_zero) * (2J + 1)^(N_down)
where J is the spin of the particle (in this case, J = 1), N_up is the number of particles with spin +1, N_zero is the number of particles with spin 0, and N_down is the number of particles with spin -1.
Since the particles are distinguishable, we need to consider the number of ways to arrange the particles among the energy levels. This is given by the multinomial coefficient:
C = N! / (N_up! * N_zero! * N_down!)
The total number of microstates for a given macrostate is then given by Ω * C.
To calculate the entropy, we use Boltzmann's entropy formula:
S = kB * ln(Ω * C)
where kB is Boltzmann's constant.
Substituting the expression for Ω * C, we have:
S = kB * ln[(2J + 1)^(N_up) * (2J + 1)^(N_zero) * (2J + 1)^(N_down) * N! / (N_up! * N_zero! * N_down!)]
Simplifying the expression, we find:
S = N * kB * ln(2J + 1) + kB * ln(N!)
The second term, kB * ln(N!), represents the combinatorial entropy of the system and is typically much smaller than the first term for large N.
Since we are given that the entropy is of the form NkBln x, we can equate the expression above with NkBln x:
N * kB * ln(2J + 1) + kB * ln(N!) = NkBln x
Cancelling out the kB terms and rearranging the equation, we find:
ln(N!) = ln x - ln(2J + 1)
The equation above implies that the value of x should be chosen such that N! is equal to (2J + 1) to the power of N. In this case, J = 1, so (2J + 1) = 3, and x = 3 is the correct answer.
To determine the entropy of the system, we need to calculate the number of microstates, Ω, that correspond to a given macrostate. The macrostate is defined by the total spin of the system, which can range from -N to +N in steps of 2.
The number of microstates for each macrostate can be determined using the formula for the degeneracy of a system with multiple particles and multiple energy levels. In this case, the energy levels correspond to the possible spin values of the particles.
The degeneracy is given by the formula:
Ω = (2J + 1)^(N_up) * (2J + 1)^(N_zero) * (2J + 1)^(N_down)
where J is the spin of the particle (in this case, J = 1), N_up is the number of particles with spin +1, N_zero is the number of particles with spin 0, and N_down is the number of particles with spin -1.
Since the particles are distinguishable, we need to consider the number of ways to arrange the particles among the energy levels. This is given by the multinomial coefficient:
C = N! / (N_up! * N_zero! * N_down!)
The total number of microstates for a given macrostate is then given by Ω * C.
To calculate the entropy, we use Boltzmann's entropy formula:
S = kB * ln(Ω * C)
where kB is Boltzmann's constant.
Substituting the expression for Ω * C, we have:
S = kB * ln[(2J + 1)^(N_up) * (2J + 1)^(N_zero) * (2J + 1)^(N_down) * N! / (N_up! * N_zero! * N_down!)]
Simplifying the expression, we find:
S = N * kB * ln(2J + 1) + kB * ln(N!)
The second term, kB * ln(N!), represents the combinatorial entropy of the system and is typically much smaller than the first term for large N.
Since we are given that the entropy is of the form NkBln x, we can equate the expression above with NkBln x:
N * kB * ln(2J + 1) + kB * ln(N!) = NkBln x
Cancelling out the kB terms and rearranging the equation, we find:
ln(N!) = ln x - ln(2J + 1)
The equation above implies that the value of x should be chosen such that N! is equal to (2J + 1) to the power of N. In this case, J = 1, so (2J + 1) = 3, and x = 3 is the correct answer.

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer?
Question Description
A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer?.
A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer?.
Solutions for A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer?, a detailed solution for A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer? has been provided alongside types of A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A system of N non-interacting and distinguishable particles of spin1 is in thermodynamic equilibrium. The entropy of the system turns out to be of the form NkBln x. Find the value of x?Correct answer is '3'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















