Physics Exam > Physics Questions > What should be the order of the energy of the...
Start Learning for Free
What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10 fm) and also nucleon charge distribution (r = 0.8 fm)(in GeV)
Correct answer is '2'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
What should be the order of the energy of the electron beam which is t...
For energy of electron,
λ ~ radius of the nucleus
= 10 fm = 10–14 m
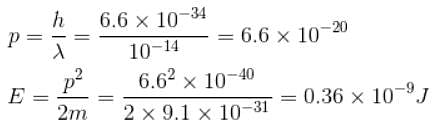
= 0.19 × 1010eV ~ 2 × 109eV
~2 GeV
The correct answer is: 2
λ ~ radius of the nucleus
= 10 fm = 10–14 m
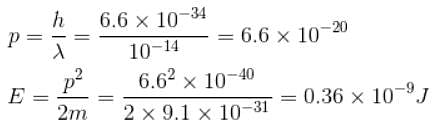
= 0.19 × 1010eV ~ 2 × 109eV
~2 GeV
The correct answer is: 2
Most Upvoted Answer
What should be the order of the energy of the electron beam which is t...
Exploring Nuclear Charge Distribution
Introduction:
When studying the charge distribution of atomic nuclei, it is important to use an appropriate electron beam energy. The energy of the electron beam determines its penetration power, which in turn affects the resolution and accuracy of the measurements. In this case, we are given two charge distributions to explore: the nuclear charge distribution with a radius of 10 fm and the nucleon charge distribution with a radius of 0.8 fm.
Determining the Electron Beam Energy:
To determine the appropriate electron beam energy, we need to consider the penetration depth of the electron beam. The penetration depth is given by the formula:
Penetration Depth = 1.44 * (hbar / (2 * m * E * (1 - (1 + E / mc^2)^(-2))))
where hbar is the reduced Planck constant, m is the electron mass, E is the electron beam energy, and c is the speed of light.
Nuclear Charge Distribution:
For the nuclear charge distribution with a radius of 10 fm, we need an electron beam energy that can penetrate through the entire nucleus. This means that the penetration depth should be greater than 10 fm.
Calculating the Electron Beam Energy for Nuclear Charge Distribution:
Let's calculate the electron beam energy required to explore the nuclear charge distribution:
Penetration Depth = 10 fm = 10 * 10^(-15) m
Plugging this value into the penetration depth formula and solving for E, we get:
E = sqrt((hbar / (1.44 * m * (1 - (1 + E / mc^2)^(-2))))^2 + (mc^2)^2) - mc^2
Nucleon Charge Distribution:
For the nucleon charge distribution with a radius of 0.8 fm, we need an electron beam energy that can penetrate through the nucleons. This means that the penetration depth should be greater than 0.8 fm.
Calculating the Electron Beam Energy for Nucleon Charge Distribution:
Let's calculate the electron beam energy required to explore the nucleon charge distribution:
Penetration Depth = 0.8 fm = 0.8 * 10^(-15) m
Plugging this value into the penetration depth formula and solving for E, we get:
E = sqrt((hbar / (1.44 * m * (1 - (1 + E / mc^2)^(-2))))^2 + (mc^2)^2) - mc^2
Comparison and Conclusion:
Comparing the two calculated electron beam energies, we find that the energy required to explore the nuclear charge distribution is greater than the energy required to explore the nucleon charge distribution.
Therefore, the correct order of the electron beam energies is 2, with the higher energy being used to explore the nuclear charge distribution (10 fm) and the lower energy being used to explore the nucleon charge distribution (0.8 fm).
Using an electron beam with the appropriate energy ensures that we can effectively study the charge distribution of atomic nuclei and gain insights into the structure of matter.
Introduction:
When studying the charge distribution of atomic nuclei, it is important to use an appropriate electron beam energy. The energy of the electron beam determines its penetration power, which in turn affects the resolution and accuracy of the measurements. In this case, we are given two charge distributions to explore: the nuclear charge distribution with a radius of 10 fm and the nucleon charge distribution with a radius of 0.8 fm.
Determining the Electron Beam Energy:
To determine the appropriate electron beam energy, we need to consider the penetration depth of the electron beam. The penetration depth is given by the formula:
Penetration Depth = 1.44 * (hbar / (2 * m * E * (1 - (1 + E / mc^2)^(-2))))
where hbar is the reduced Planck constant, m is the electron mass, E is the electron beam energy, and c is the speed of light.
Nuclear Charge Distribution:
For the nuclear charge distribution with a radius of 10 fm, we need an electron beam energy that can penetrate through the entire nucleus. This means that the penetration depth should be greater than 10 fm.
Calculating the Electron Beam Energy for Nuclear Charge Distribution:
Let's calculate the electron beam energy required to explore the nuclear charge distribution:
Penetration Depth = 10 fm = 10 * 10^(-15) m
Plugging this value into the penetration depth formula and solving for E, we get:
E = sqrt((hbar / (1.44 * m * (1 - (1 + E / mc^2)^(-2))))^2 + (mc^2)^2) - mc^2
Nucleon Charge Distribution:
For the nucleon charge distribution with a radius of 0.8 fm, we need an electron beam energy that can penetrate through the nucleons. This means that the penetration depth should be greater than 0.8 fm.
Calculating the Electron Beam Energy for Nucleon Charge Distribution:
Let's calculate the electron beam energy required to explore the nucleon charge distribution:
Penetration Depth = 0.8 fm = 0.8 * 10^(-15) m
Plugging this value into the penetration depth formula and solving for E, we get:
E = sqrt((hbar / (1.44 * m * (1 - (1 + E / mc^2)^(-2))))^2 + (mc^2)^2) - mc^2
Comparison and Conclusion:
Comparing the two calculated electron beam energies, we find that the energy required to explore the nuclear charge distribution is greater than the energy required to explore the nucleon charge distribution.
Therefore, the correct order of the electron beam energies is 2, with the higher energy being used to explore the nuclear charge distribution (10 fm) and the lower energy being used to explore the nucleon charge distribution (0.8 fm).
Using an electron beam with the appropriate energy ensures that we can effectively study the charge distribution of atomic nuclei and gain insights into the structure of matter.

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer?
Question Description
What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer?.
What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer?.
Solutions for What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer?, a detailed solution for What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer? has been provided alongside types of What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice What should be the order of the energy of the electron beam which is to be used to explore the nuclear charge distribution (take the radius to be of the order of 10fm) and also nucleon charge distribution (r= 0.8fm)(inGeV)Correct answer is '2'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















