Physics Exam > Physics Questions > The property of a working substance which inc...
Start Learning for Free
The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.
Select one:
Select one:
- a)internal energy
- b)entropy
- c)external energy
- d)enthalpy
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The property of a working substance which increases or decreases as th...
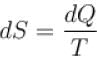
Hence, entropy increases or decreases as heat is supplied or removed in reversible manner.
The correct answer is: entropy
Most Upvoted Answer
The property of a working substance which increases or decreases as th...
Entropy is the property of a working substance which increases or decreases as heat is supplied or removed in a reversible manner. It is a measure of the randomness or disorder in a system.
Explanation:
1. Reversible Process:
In order to understand the concept of entropy, it is important to grasp the idea of a reversible process. A reversible process is one in which the system and its surroundings can be restored to their original state by reversing the process. It is an idealized process that does not occur in nature, but it serves as a useful theoretical concept.
2. Heat Transfer and Entropy:
When heat is supplied to a system, the energy is transferred from a higher temperature region to a lower temperature region. This transfer of energy increases the entropy of the system. Conversely, when heat is removed from a system, the energy is transferred from a lower temperature region to a higher temperature region, resulting in a decrease in entropy.
3. Randomness and Disorder:
Entropy is often described as a measure of the randomness or disorder in a system. When heat is added to a system, the molecules of the working substance gain energy and move more randomly, increasing the disorder. This increase in disorder is reflected in the increase in entropy.
4. Internal Energy vs Entropy:
While internal energy (option A) is a measure of the total energy of a system, entropy (option B) is a measure of the disorder or randomness in a system. Internal energy does not necessarily change with the addition or removal of heat, but entropy does.
5. External Energy:
External energy (option C) does not directly relate to the increase or decrease of entropy. It refers to the energy associated with the system's surroundings, such as potential energy or kinetic energy. It is not the property that changes with the addition or removal of heat in a reversible manner.
6. Enthalpy:
Enthalpy (option D) is a measure of the heat content of a system at constant pressure. It is related to the internal energy of a system, but it does not directly relate to the increase or decrease of entropy.
Therefore, the property of a working substance which increases or decreases as heat is supplied or removed in a reversible manner is known as entropy.
Explanation:
1. Reversible Process:
In order to understand the concept of entropy, it is important to grasp the idea of a reversible process. A reversible process is one in which the system and its surroundings can be restored to their original state by reversing the process. It is an idealized process that does not occur in nature, but it serves as a useful theoretical concept.
2. Heat Transfer and Entropy:
When heat is supplied to a system, the energy is transferred from a higher temperature region to a lower temperature region. This transfer of energy increases the entropy of the system. Conversely, when heat is removed from a system, the energy is transferred from a lower temperature region to a higher temperature region, resulting in a decrease in entropy.
3. Randomness and Disorder:
Entropy is often described as a measure of the randomness or disorder in a system. When heat is added to a system, the molecules of the working substance gain energy and move more randomly, increasing the disorder. This increase in disorder is reflected in the increase in entropy.
4. Internal Energy vs Entropy:
While internal energy (option A) is a measure of the total energy of a system, entropy (option B) is a measure of the disorder or randomness in a system. Internal energy does not necessarily change with the addition or removal of heat, but entropy does.
5. External Energy:
External energy (option C) does not directly relate to the increase or decrease of entropy. It refers to the energy associated with the system's surroundings, such as potential energy or kinetic energy. It is not the property that changes with the addition or removal of heat in a reversible manner.
6. Enthalpy:
Enthalpy (option D) is a measure of the heat content of a system at constant pressure. It is related to the internal energy of a system, but it does not directly relate to the increase or decrease of entropy.
Therefore, the property of a working substance which increases or decreases as heat is supplied or removed in a reversible manner is known as entropy.

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer?
Question Description
The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer?.
The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer?.
Solutions for The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.Select one:a)internal energyb)entropyc)external energyd)enthalpyCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















