JEE Exam > JEE Questions > Sodium thiosulphate is used in photography be...
Start Learning for Free
Sodium thiosulphate is used in photography because of its
- a)reducing behaviour
- b)oxidising behaviour
- c)complex forming behaviour
- d)reaction with light
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Sodium thiosulphate is used in photography because of itsa)reducing be...
Hypo solution (Na2S2O3) is used in photography to remove the unaffected AgBr in the form of soluble complex.
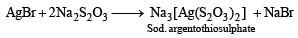
Most Upvoted Answer
Sodium thiosulphate is used in photography because of itsa)reducing be...
Complex Forming Behavior of Sodium Thiosulphate in Photography:
Photography involves the use of light to capture images on photosensitive materials such as film or photographic paper. Sodium thiosulphate, also known as hypo, is commonly used in photography for its unique complex forming behavior.
Role in Fixing Process:
- Sodium thiosulphate is used in the fixing process of traditional film photography.
- During development, unexposed silver halide crystals are removed from the film or paper.
- The remaining silver halide crystals that formed the image are then made soluble by the sodium thiosulphate.
Formation of Complex with Silver Ions:
- Sodium thiosulphate forms a complex with the silver ions present in the silver halide crystals.
- This complex is soluble in water, allowing the unexposed silver halide crystals to be washed away, leaving behind the developed image.
Preventing Further Exposure:
- Sodium thiosulphate also helps in preventing further exposure of the image to light.
- By removing the unexposed silver halide crystals, it ensures that the image is fixed and does not continue to darken upon exposure to light.
Conclusion:
In conclusion, sodium thiosulphate's complex forming behavior plays a crucial role in the fixing process of traditional photography. It helps in removing unexposed silver halide crystals, forming soluble complexes with silver ions, and preventing further exposure of the developed image to light.
Photography involves the use of light to capture images on photosensitive materials such as film or photographic paper. Sodium thiosulphate, also known as hypo, is commonly used in photography for its unique complex forming behavior.
Role in Fixing Process:
- Sodium thiosulphate is used in the fixing process of traditional film photography.
- During development, unexposed silver halide crystals are removed from the film or paper.
- The remaining silver halide crystals that formed the image are then made soluble by the sodium thiosulphate.
Formation of Complex with Silver Ions:
- Sodium thiosulphate forms a complex with the silver ions present in the silver halide crystals.
- This complex is soluble in water, allowing the unexposed silver halide crystals to be washed away, leaving behind the developed image.
Preventing Further Exposure:
- Sodium thiosulphate also helps in preventing further exposure of the image to light.
- By removing the unexposed silver halide crystals, it ensures that the image is fixed and does not continue to darken upon exposure to light.
Conclusion:
In conclusion, sodium thiosulphate's complex forming behavior plays a crucial role in the fixing process of traditional photography. It helps in removing unexposed silver halide crystals, forming soluble complexes with silver ions, and preventing further exposure of the developed image to light.

|
Explore Courses for JEE exam
|

|
Question Description
Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer?.
Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Sodium thiosulphate is used in photography because of itsa)reducing behaviourb)oxidising behaviourc)complex forming behaviourd)reaction with lightCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























