Physics Exam > Physics Questions > The longest wavelength in the Balmer series o...
Start Learning for Free
The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?
- a)121 nm
- b)156 nm
- c)243 nm
- d)276 nm
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The longest wavelength in the Balmer series of hydrogen atom is given ...
The expression of wavelength emitted when an electron makes a transition from nth level to mth level is given by
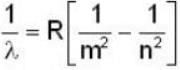
for Balmer series m = 2

The value for n for longest wavelength will be 3
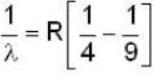
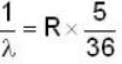
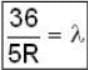
for positronium mass m will be replaced with reduced mass μ.
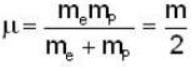
Rydberg constant for H - atom
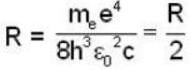
Rydberg constant for positronium
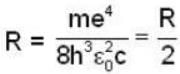
for lyman series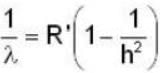
for longest wavelength n = 2
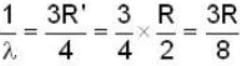
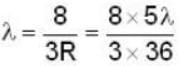
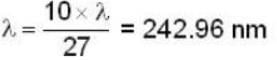
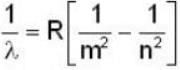
for Balmer series m = 2

The value for n for longest wavelength will be 3
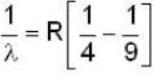
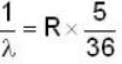

for positronium mass m will be replaced with reduced mass μ.
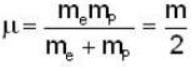
Rydberg constant for H - atom
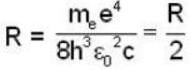
Rydberg constant for positronium
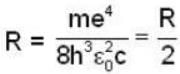
for lyman series
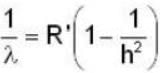
for longest wavelength n = 2
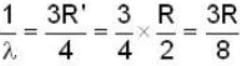
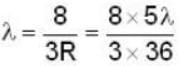
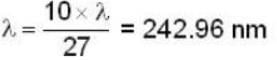
Most Upvoted Answer
The longest wavelength in the Balmer series of hydrogen atom is given ...
Understanding Positronium
Positronium is a unique atom-like structure formed by an electron and its antiparticle, the positron. Its behavior is similar to hydrogen, but due to the reduced mass of the system, the energy levels differ.
Key Concept: Energy Levels
- The energy levels of positronium can be calculated using the formula similar to hydrogen:
- E_n = - (μ * Z^2 * e^4) / (2 * n^2 * h^2)
- Here, μ is the reduced mass, Z is the atomic number, and n is the principal quantum number.
Reduced Mass of Positronium
- The reduced mass (μ) of positronium is given by:
- μ = (m_e * m_p) / (m_e + m_p)
- Since m_e = m_p (mass of electron and positron are equal), we have:
- μ = m_e / 2
Comparison with Hydrogen
- In hydrogen, the longest wavelength in the Lyman series (n=2 to n=1 transition) corresponds to 121 nm.
- The wavelength (λ) is inversely proportional to the energy difference between the levels.
Calculating the Wavelength for Positronium
- Since the reduced mass of positronium is half that of hydrogen, the energy levels will be halved.
- Therefore, the wavelength for the transition from n=2 to n=1 in positronium will be twice that of hydrogen:
- λ_pos = 2 * λ_Hydrogen = 2 * 121 nm = 242 nm.
Conclusion
- The longest wavelength in the Lyman series for positronium is approximately 243 nm (option C).
- This demonstrates the distinct behavior of positronium compared to hydrogen due to its unique particle composition.
Positronium is a unique atom-like structure formed by an electron and its antiparticle, the positron. Its behavior is similar to hydrogen, but due to the reduced mass of the system, the energy levels differ.
Key Concept: Energy Levels
- The energy levels of positronium can be calculated using the formula similar to hydrogen:
- E_n = - (μ * Z^2 * e^4) / (2 * n^2 * h^2)
- Here, μ is the reduced mass, Z is the atomic number, and n is the principal quantum number.
Reduced Mass of Positronium
- The reduced mass (μ) of positronium is given by:
- μ = (m_e * m_p) / (m_e + m_p)
- Since m_e = m_p (mass of electron and positron are equal), we have:
- μ = m_e / 2
Comparison with Hydrogen
- In hydrogen, the longest wavelength in the Lyman series (n=2 to n=1 transition) corresponds to 121 nm.
- The wavelength (λ) is inversely proportional to the energy difference between the levels.
Calculating the Wavelength for Positronium
- Since the reduced mass of positronium is half that of hydrogen, the energy levels will be halved.
- Therefore, the wavelength for the transition from n=2 to n=1 in positronium will be twice that of hydrogen:
- λ_pos = 2 * λ_Hydrogen = 2 * 121 nm = 242 nm.
Conclusion
- The longest wavelength in the Lyman series for positronium is approximately 243 nm (option C).
- This demonstrates the distinct behavior of positronium compared to hydrogen due to its unique particle composition.
Free Test
FREE
| Start Free Test |
Community Answer
The longest wavelength in the Balmer series of hydrogen atom is given ...
The expression of wavelength emitted when an electron makes a transition from nth level to mth level is given by
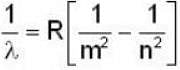
for Balmer series m = 2
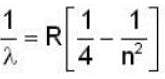
The value for n for longest wavelength will be 3
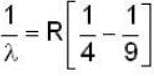
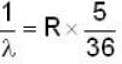
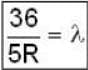
for positronium mass m will be replaced with reduced mass μ.
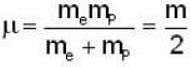
Rydberg constant for H - atom
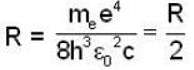
Rydberg constant for positronium
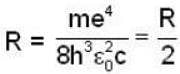
for lyman series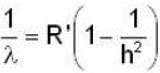
for longest wavelength n = 2
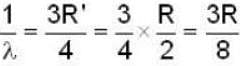
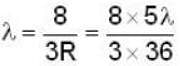
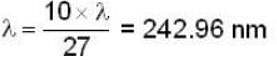
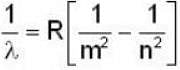
for Balmer series m = 2
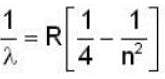
The value for n for longest wavelength will be 3
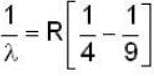
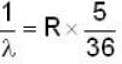

for positronium mass m will be replaced with reduced mass μ.
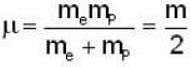
Rydberg constant for H - atom
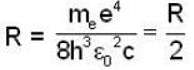
Rydberg constant for positronium
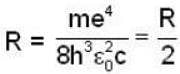
for lyman series
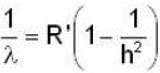
for longest wavelength n = 2
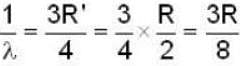
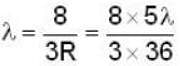
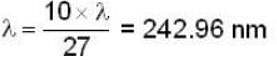

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer?
Question Description
The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer?.
The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer?.
Solutions for The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?a)121 nmb)156 nmc)243 nmd)276 nmCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















