IIT JAM Exam > IIT JAM Questions > If the ratio of ATP: ADP is 105 :1, the Gibbs...
Start Learning for Free
If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)
Correct answer is between '-60,-59'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hy...
This question can be solved using the formula ΔG =ΔGº + 2.303 RT log 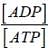
ΔG = -30.000 + 2.303 x 8.31 x 310 x log 10-5
ΔG = -30.000 + 5933 x logl0-5
ΔG = -30000 + 5933 x (-5)
ΔG = -30000 - 29665
ΔG = -59665 = - 59.665 kJ = - 60 kJ
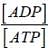
ΔG = -30.000 + 2.303 x 8.31 x 310 x log 10-5
ΔG = -30.000 + 5933 x logl0-5
ΔG = -30000 + 5933 x (-5)
ΔG = -30000 - 29665
ΔG = -59665 = - 59.665 kJ = - 60 kJ
Most Upvoted Answer
If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hy...
°C and pH 7.0 is -7.3 kcal/mol.
The Gibbs free energy change of hydrolysis of ADP can be calculated using the following equation:
ΔG° = ΔG°' + RT ln([ADP]/[ATP][Pi])
Where:
ΔG° = standard Gibbs free energy change (-7.3 kcal/mol)
ΔG°' = standard Gibbs free energy change of hydrolysis of ADP (unknown)
R = gas constant (1.987 cal/mol·K)
T = absolute temperature (37°C + 273 = 310 K)
ln = natural logarithm
[ADP], [ATP], and [Pi] = concentrations of ADP, ATP, and inorganic phosphate (Pi), respectively
Assuming that the concentration of Pi is negligible compared to ATP and ADP, the equation simplifies to:
ΔG° = ΔG°' + RT ln([ADP]/[ATP])
Substituting the given values:
-7.3 = ΔG°' + (1.987)(310) ln([1]/[105])
Solving for ΔG°':
ΔG°' = -7.3 - (1.987)(310) ln([1]/[105])
ΔG°' = -7.3 + 12.9
ΔG°' = 5.6 kcal/mol
Therefore, the standard Gibbs free energy change of hydrolysis of ADP is 5.6 kcal/mol.
The Gibbs free energy change of hydrolysis of ADP can be calculated using the following equation:
ΔG° = ΔG°' + RT ln([ADP]/[ATP][Pi])
Where:
ΔG° = standard Gibbs free energy change (-7.3 kcal/mol)
ΔG°' = standard Gibbs free energy change of hydrolysis of ADP (unknown)
R = gas constant (1.987 cal/mol·K)
T = absolute temperature (37°C + 273 = 310 K)
ln = natural logarithm
[ADP], [ATP], and [Pi] = concentrations of ADP, ATP, and inorganic phosphate (Pi), respectively
Assuming that the concentration of Pi is negligible compared to ATP and ADP, the equation simplifies to:
ΔG° = ΔG°' + RT ln([ADP]/[ATP])
Substituting the given values:
-7.3 = ΔG°' + (1.987)(310) ln([1]/[105])
Solving for ΔG°':
ΔG°' = -7.3 - (1.987)(310) ln([1]/[105])
ΔG°' = -7.3 + 12.9
ΔG°' = 5.6 kcal/mol
Therefore, the standard Gibbs free energy change of hydrolysis of ADP is 5.6 kcal/mol.

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer?
Question Description
If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer?.
If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer?.
Solutions for If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer?, a detailed solution for If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer? has been provided alongside types of If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice If the ratio of ATP: ADP is 105 :1, the Gibbs free energy change of hydrolysis of ATP at 37°C will be _____ (kilo Joule). Given that standard Gibbs free energy change is –30 kJ/ mol. (Answer in integer)Correct answer is between '-60,-59'. Can you explain this answer? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















