IIT JAM Exam > IIT JAM Questions > Osazone are formed by a chemical reaction bet...
Start Learning for Free
Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group and
- a)Phenyl hydrazine
- b)Sulphuric acid
- c)Mercaptoethanol
- d)Hydroxylamine
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
Osazone are formed by a chemical reaction between carbohydrates contai...
Carbohydrates reaction with phenyl hydrazine leads to the formation of Osazone.
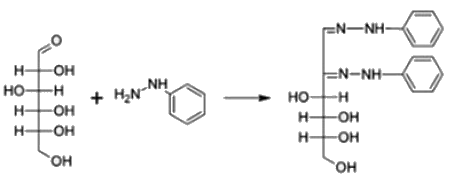
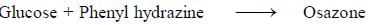
All reducing sugars form osazones. Glucose is a reducing sugar hence it forms osazone. Sucrose does not form osazone crystals because it is a non-reducing sugar as it has no free carbonyl group. Sucrose is made
up of glucose and fructose. The linkage in sucrose is Glucose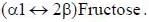

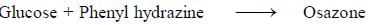
All reducing sugars form osazones. Glucose is a reducing sugar hence it forms osazone. Sucrose does not form osazone crystals because it is a non-reducing sugar as it has no free carbonyl group. Sucrose is made
up of glucose and fructose. The linkage in sucrose is Glucose
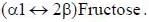
Most Upvoted Answer
Osazone are formed by a chemical reaction between carbohydrates contai...
Formation of Osazones:
Osazones are crystalline compounds that are formed by the condensation reaction between carbohydrates containing a free aldehyde or keto group and phenylhydrazine. This reaction is known as the osazone formation reaction and is widely used for the identification and characterization of carbohydrates.
Reaction Mechanism:
The reaction between a carbohydrate and phenylhydrazine involves the following steps:
1. Formation of Schiff's base: The first step is the formation of a Schiff's base between the carbonyl group of the carbohydrate and the phenylhydrazine. This is a nucleophilic addition reaction where the lone pair of electrons on the nitrogen atom of phenylhydrazine attacks the carbonyl carbon of the carbohydrate. This results in the formation of an imine or Schiff's base.
2. Conversion to osazone: The imine formed in the previous step undergoes further reaction with another molecule of phenylhydrazine. This leads to the formation of a hydrazone. The hydrazone then undergoes a condensation reaction with the elimination of water molecule to form the osazone.
Role of Phenylhydrazine:
Phenylhydrazine is a strong nucleophile due to the presence of the lone pair of electrons on the nitrogen atom. It can attack the carbonyl carbon of the carbohydrate, leading to the formation of a Schiff's base. Phenylhydrazine also acts as a reducing agent, converting the aldehyde or keto group of the carbohydrate into an imine or hydrazone, respectively.
Role of Sulphuric Acid, Mercaptoethanol, and Hydroxylamine:
Sulphuric acid, mercaptoethanol, and hydroxylamine are not involved in the formation of osazones. They may have other roles in different chemical reactions or processes. However, in the context of osazone formation, the correct answer is option 'A' - Phenylhydrazine.
Overall, osazones are formed by the chemical reaction between carbohydrates containing a free aldehyde or keto group and phenylhydrazine. Phenylhydrazine acts as a nucleophile and reducing agent, leading to the formation of Schiff's base and subsequent condensation to form the osazone.
Osazones are crystalline compounds that are formed by the condensation reaction between carbohydrates containing a free aldehyde or keto group and phenylhydrazine. This reaction is known as the osazone formation reaction and is widely used for the identification and characterization of carbohydrates.
Reaction Mechanism:
The reaction between a carbohydrate and phenylhydrazine involves the following steps:
1. Formation of Schiff's base: The first step is the formation of a Schiff's base between the carbonyl group of the carbohydrate and the phenylhydrazine. This is a nucleophilic addition reaction where the lone pair of electrons on the nitrogen atom of phenylhydrazine attacks the carbonyl carbon of the carbohydrate. This results in the formation of an imine or Schiff's base.
2. Conversion to osazone: The imine formed in the previous step undergoes further reaction with another molecule of phenylhydrazine. This leads to the formation of a hydrazone. The hydrazone then undergoes a condensation reaction with the elimination of water molecule to form the osazone.
Role of Phenylhydrazine:
Phenylhydrazine is a strong nucleophile due to the presence of the lone pair of electrons on the nitrogen atom. It can attack the carbonyl carbon of the carbohydrate, leading to the formation of a Schiff's base. Phenylhydrazine also acts as a reducing agent, converting the aldehyde or keto group of the carbohydrate into an imine or hydrazone, respectively.
Role of Sulphuric Acid, Mercaptoethanol, and Hydroxylamine:
Sulphuric acid, mercaptoethanol, and hydroxylamine are not involved in the formation of osazones. They may have other roles in different chemical reactions or processes. However, in the context of osazone formation, the correct answer is option 'A' - Phenylhydrazine.
Overall, osazones are formed by the chemical reaction between carbohydrates containing a free aldehyde or keto group and phenylhydrazine. Phenylhydrazine acts as a nucleophile and reducing agent, leading to the formation of Schiff's base and subsequent condensation to form the osazone.

|
Explore Courses for IIT JAM exam
|

|
Question Description
Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer? for IIT JAM 2025 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for IIT JAM 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer?.
Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer? for IIT JAM 2025 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for IIT JAM 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Osazone are formed by a chemical reaction between carbohydrates containing a free aldehyde or keto group anda)Phenyl hydrazineb)Sulphuric acidc)Mercaptoethanold)HydroxylamineCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.