Chemistry Exam > Chemistry Questions > The bond that gives the most intense band in ...
Start Learning for Free
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:
- a)C—H
- b)N—H
- c)O—H
- d)S—H
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
The bond that gives the most intense band in the infrared spectrum for...
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is the OH bond.
Explanation:
- Infrared spectroscopy is a technique used to identify and analyze the functional groups present in a molecule by measuring the absorption of infrared radiation by the sample.
- When a molecule absorbs infrared radiation, it causes the bonds to vibrate and stretch. The frequency of the vibration is related to the strength of the bond and the mass of the atoms involved.
- The stretching vibrations of the OH bond are known to give rise to the most intense band in the infrared spectrum due to the following reasons:
- The OH bond is polar, and it has a large dipole moment. This dipole moment makes the bond more susceptible to infrared radiation absorption.
- The OH bond is relatively weak compared to other bonds, such as CH and NH bonds. Therefore, it requires less energy to stretch and vibrate, making it more likely to absorb infrared radiation.
- The OH group is present in many important functional groups, such as alcohols, phenols, and carboxylic acids. Therefore, the intense band in the infrared spectrum due to OH stretching vibrations can be used to identify these functional groups in a molecule.
- In addition, the position and shape of the OH band in the infrared spectrum can provide information about the hydrogen bonding and the environment surrounding the OH group.
In conclusion, the OH bond gives the most intense band in the infrared spectrum for its stretching vibrations due to its polarity, weak strength, and prevalence in important functional groups.
Explanation:
- Infrared spectroscopy is a technique used to identify and analyze the functional groups present in a molecule by measuring the absorption of infrared radiation by the sample.
- When a molecule absorbs infrared radiation, it causes the bonds to vibrate and stretch. The frequency of the vibration is related to the strength of the bond and the mass of the atoms involved.
- The stretching vibrations of the OH bond are known to give rise to the most intense band in the infrared spectrum due to the following reasons:
- The OH bond is polar, and it has a large dipole moment. This dipole moment makes the bond more susceptible to infrared radiation absorption.
- The OH bond is relatively weak compared to other bonds, such as CH and NH bonds. Therefore, it requires less energy to stretch and vibrate, making it more likely to absorb infrared radiation.
- The OH group is present in many important functional groups, such as alcohols, phenols, and carboxylic acids. Therefore, the intense band in the infrared spectrum due to OH stretching vibrations can be used to identify these functional groups in a molecule.
- In addition, the position and shape of the OH band in the infrared spectrum can provide information about the hydrogen bonding and the environment surrounding the OH group.
In conclusion, the OH bond gives the most intense band in the infrared spectrum for its stretching vibrations due to its polarity, weak strength, and prevalence in important functional groups.
Free Test
FREE
| Start Free Test |
Community Answer
The bond that gives the most intense band in the infrared spectrum for...
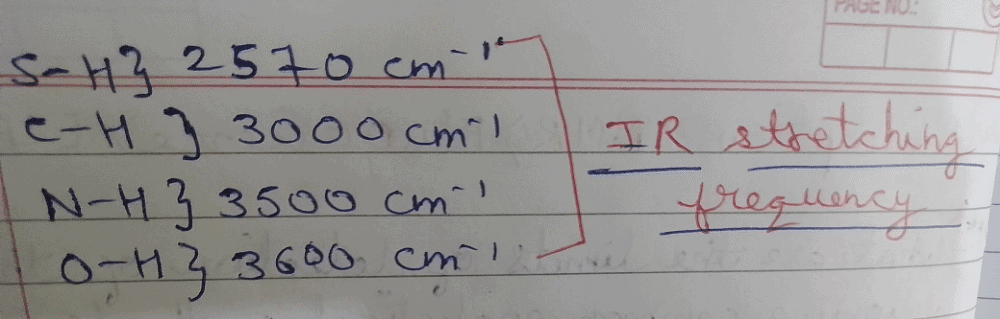

|
Explore Courses for Chemistry exam
|

|
Question Description
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer?.
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer?.
Solutions for The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The bond that gives the most intense band in the infrared spectrum for its stretching vibrations is:a)C—Hb)N—Hc)O—Hd)S—HCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















