Class 10 Exam > Class 10 Questions > What is the electron dot structure of calcium...
Start Learning for Free
What is the electron dot structure of calcium chloride?
Most Upvoted Answer
What is the electron dot structure of calcium chloride?
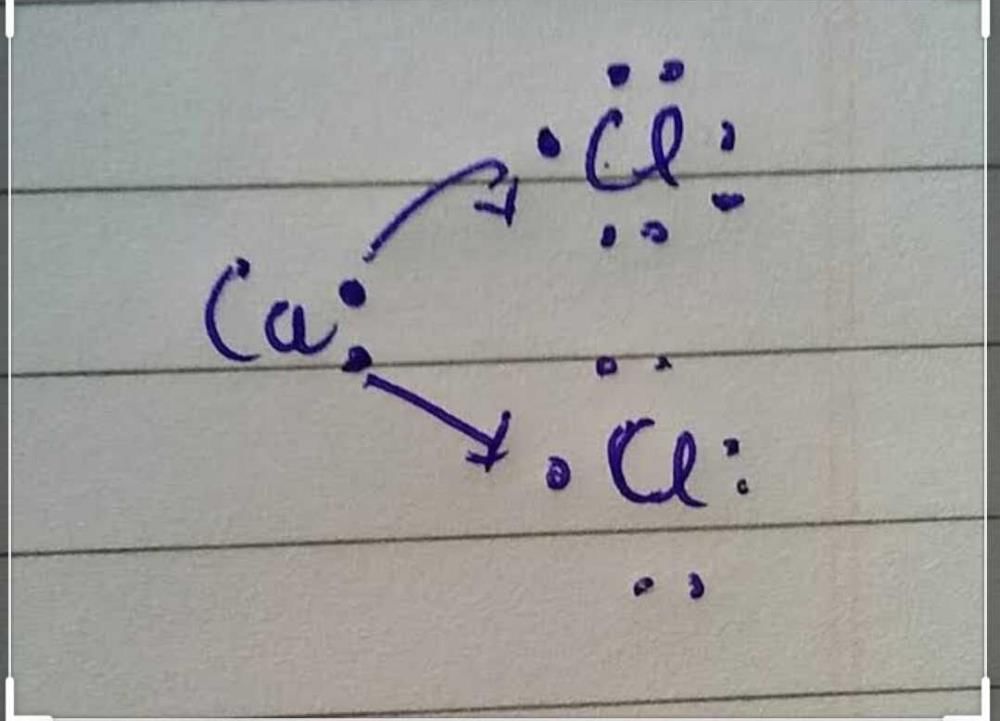
Community Answer
What is the electron dot structure of calcium chloride?
Electron Dot Structure of Calcium Chloride (CaCl2)
Calcium chloride (CaCl2) is an ionic compound composed of calcium (Ca) and chloride (Cl) ions. To represent the electron dot structure of calcium chloride, we need to follow certain rules and guidelines.
Step 1: Determine the Number of Valence Electrons
The first step is to determine the number of valence electrons for each atom in the compound. Valence electrons are the electrons in the outermost energy level of an atom.
- Calcium (Ca) is in Group 2 of the periodic table, so it has 2 valence electrons.
- Chlorine (Cl) is in Group 17, so it has 7 valence electrons.
Step 2: Represent the Atoms
Next, we represent the atoms using their chemical symbols.
- Calcium is represented by the symbol Ca.
- Chlorine is represented by the symbol Cl.
Step 3: Draw the Electron Dot Structure
To draw the electron dot structure, we place the valence electrons around the chemical symbol, representing each electron with a dot. The dots are placed around the symbol, one at a time, following a specific pattern.
- The first four dots are placed on each side of the chemical symbol, representing the s and p orbitals.
- Each side can accommodate a maximum of two electrons.
- Once all four sides have two electrons, we start pairing the remaining electrons on the existing sides.
Step 4: Complete the Electron Dot Structure
Using the above guidelines, we can now draw the electron dot structure of calcium chloride (CaCl2).
- Calcium (Ca) has 2 valence electrons, so we place two dots next to the symbol Ca.
- Chlorine (Cl) has 7 valence electrons, so we place one dot next to each symbol Cl.
The complete electron dot structure of calcium chloride (CaCl2) is as follows:
Ca
:
Cl : Cl
Summary
To summarize, the electron dot structure of calcium chloride (CaCl2) is represented by placing two dots next to the symbol Ca to represent its 2 valence electrons and one dot next to each symbol Cl to represent their 7 valence electrons.
Calcium chloride (CaCl2) is an ionic compound composed of calcium (Ca) and chloride (Cl) ions. To represent the electron dot structure of calcium chloride, we need to follow certain rules and guidelines.
Step 1: Determine the Number of Valence Electrons
The first step is to determine the number of valence electrons for each atom in the compound. Valence electrons are the electrons in the outermost energy level of an atom.
- Calcium (Ca) is in Group 2 of the periodic table, so it has 2 valence electrons.
- Chlorine (Cl) is in Group 17, so it has 7 valence electrons.
Step 2: Represent the Atoms
Next, we represent the atoms using their chemical symbols.
- Calcium is represented by the symbol Ca.
- Chlorine is represented by the symbol Cl.
Step 3: Draw the Electron Dot Structure
To draw the electron dot structure, we place the valence electrons around the chemical symbol, representing each electron with a dot. The dots are placed around the symbol, one at a time, following a specific pattern.
- The first four dots are placed on each side of the chemical symbol, representing the s and p orbitals.
- Each side can accommodate a maximum of two electrons.
- Once all four sides have two electrons, we start pairing the remaining electrons on the existing sides.
Step 4: Complete the Electron Dot Structure
Using the above guidelines, we can now draw the electron dot structure of calcium chloride (CaCl2).
- Calcium (Ca) has 2 valence electrons, so we place two dots next to the symbol Ca.
- Chlorine (Cl) has 7 valence electrons, so we place one dot next to each symbol Cl.
The complete electron dot structure of calcium chloride (CaCl2) is as follows:
Ca
:
Cl : Cl
Summary
To summarize, the electron dot structure of calcium chloride (CaCl2) is represented by placing two dots next to the symbol Ca to represent its 2 valence electrons and one dot next to each symbol Cl to represent their 7 valence electrons.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
What is the electron dot structure of calcium chloride?
Question Description
What is the electron dot structure of calcium chloride? for Class 10 2025 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about What is the electron dot structure of calcium chloride? covers all topics & solutions for Class 10 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the electron dot structure of calcium chloride?.
What is the electron dot structure of calcium chloride? for Class 10 2025 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about What is the electron dot structure of calcium chloride? covers all topics & solutions for Class 10 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the electron dot structure of calcium chloride?.
Solutions for What is the electron dot structure of calcium chloride? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of What is the electron dot structure of calcium chloride? defined & explained in the simplest way possible. Besides giving the explanation of
What is the electron dot structure of calcium chloride?, a detailed solution for What is the electron dot structure of calcium chloride? has been provided alongside types of What is the electron dot structure of calcium chloride? theory, EduRev gives you an
ample number of questions to practice What is the electron dot structure of calcium chloride? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



























