Mechanical Engineering Exam > Mechanical Engineering Questions > The Clausius-Clapeyron equation gives the slo...
Start Learning for Free
The Clausius-Clapeyron equation gives the slope of curve on
- a)P - V plot
- b)P - h plot
- c)P - T plot
- d)T - s plot
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
The Clausius-Clapeyron equation gives the slope of curve ona)P - V plo...
Most Upvoted Answer
The Clausius-Clapeyron equation gives the slope of curve ona)P - V plo...
Clausius-Clapeyron Equation in P-T Plot:
The Clausius-Clapeyron equation is a fundamental equation in thermodynamics that relates the vapor pressure of a substance to its temperature. It provides a mathematical relationship that describes the behavior of the phase equilibrium curve between the liquid and vapor phases of a substance.
The equation is expressed as:
ln(P2/P1) = (ΔHvap/R)(1/T1 - 1/T2)
where P1 and P2 are the vapor pressures of the substance at temperatures T1 and T2 respectively, ΔHvap is the enthalpy of vaporization, R is the gas constant, and T1 and T2 are the temperatures in Kelvin.
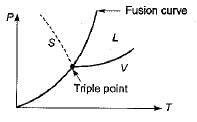
The Clausius-Clapeyron equation is particularly useful when representing the phase equilibrium curve on a P-T plot. In this plot, the pressure (P) is plotted on the y-axis, and the temperature (T) is plotted on the x-axis.
Explanation:
The correct answer is option 'C' because the Clausius-Clapeyron equation gives the slope of the curve on a P-T plot. Let's understand why:
1. P-T plot:
- The P-T plot is a graph that represents the behavior of a substance as it undergoes a phase change from a liquid to a vapor.
- The pressure (P) is plotted on the y-axis, and the temperature (T) is plotted on the x-axis.
- The phase equilibrium curve on the P-T plot represents the conditions at which the substance exists in equilibrium between the liquid and vapor phases.
2. Clausius-Clapeyron equation:
- The Clausius-Clapeyron equation relates the vapor pressure of a substance to its temperature.
- It provides a mathematical relationship that describes the behavior of the phase equilibrium curve.
- The equation gives the slope of the curve on the P-T plot, representing how the vapor pressure changes with temperature.
3. Slope interpretation:
- The slope of a curve represents the rate at which one variable changes with respect to another variable.
- In the context of the Clausius-Clapeyron equation, the slope represents the rate at which the vapor pressure changes with temperature.
- A steeper slope indicates a higher rate of change in vapor pressure with temperature, while a shallower slope indicates a lower rate of change.
Therefore, the Clausius-Clapeyron equation is used to determine the slope of the phase equilibrium curve on a P-T plot, making option 'C' the correct answer.
The Clausius-Clapeyron equation is a fundamental equation in thermodynamics that relates the vapor pressure of a substance to its temperature. It provides a mathematical relationship that describes the behavior of the phase equilibrium curve between the liquid and vapor phases of a substance.
The equation is expressed as:
ln(P2/P1) = (ΔHvap/R)(1/T1 - 1/T2)
where P1 and P2 are the vapor pressures of the substance at temperatures T1 and T2 respectively, ΔHvap is the enthalpy of vaporization, R is the gas constant, and T1 and T2 are the temperatures in Kelvin.
The Clausius-Clapeyron equation is particularly useful when representing the phase equilibrium curve on a P-T plot. In this plot, the pressure (P) is plotted on the y-axis, and the temperature (T) is plotted on the x-axis.
Explanation:
The correct answer is option 'C' because the Clausius-Clapeyron equation gives the slope of the curve on a P-T plot. Let's understand why:
1. P-T plot:
- The P-T plot is a graph that represents the behavior of a substance as it undergoes a phase change from a liquid to a vapor.
- The pressure (P) is plotted on the y-axis, and the temperature (T) is plotted on the x-axis.
- The phase equilibrium curve on the P-T plot represents the conditions at which the substance exists in equilibrium between the liquid and vapor phases.
2. Clausius-Clapeyron equation:
- The Clausius-Clapeyron equation relates the vapor pressure of a substance to its temperature.
- It provides a mathematical relationship that describes the behavior of the phase equilibrium curve.
- The equation gives the slope of the curve on the P-T plot, representing how the vapor pressure changes with temperature.
3. Slope interpretation:
- The slope of a curve represents the rate at which one variable changes with respect to another variable.
- In the context of the Clausius-Clapeyron equation, the slope represents the rate at which the vapor pressure changes with temperature.
- A steeper slope indicates a higher rate of change in vapor pressure with temperature, while a shallower slope indicates a lower rate of change.
Therefore, the Clausius-Clapeyron equation is used to determine the slope of the phase equilibrium curve on a P-T plot, making option 'C' the correct answer.

|
Explore Courses for Mechanical Engineering exam
|

|
Question Description
The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer?.
The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer?.
Solutions for The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The Clausius-Clapeyron equation gives the slope of curve ona)P - V plotb)P - hplotc)P - T plotd)T - s plotCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















