Class 12 Exam > Class 12 Questions > For conversion of bromobenzene to benzoic aci...
Start Learning for Free
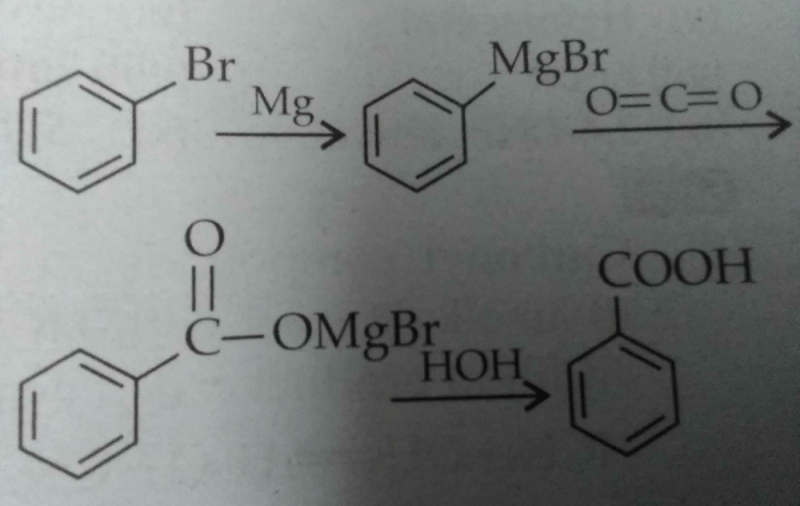
For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead?
Most Upvoted Answer
For conversion of bromobenzene to benzoic acid the given method is giv...
Conversion of Bromobenzene to Benzoic Acid
Bromobenzene can indeed be converted to benzoic acid using potassium cyanide (KCN) followed by hydrolysis. Here’s how the process works:
Step 1: Nucleophilic Substitution
- Bromobenzene contains a bromine atom, which is a good leaving group.
- When treated with KCN, a nucleophilic substitution reaction occurs.
- The cyanide ion (CN-) acts as a nucleophile, attacking the carbon atom bonded to bromine.
- This results in the formation of phenyl cyanide (benzonitrile).
Step 2: Hydrolysis
- The next step involves hydrolysis of the benzonitrile.
- Hydrolysis can be performed using aqueous acid (HCl) or a base (NaOH) under heat.
- In this reaction, the nitrile group (−C≡N) is converted to a carboxylic acid group (−COOH).
- This results in the formation of benzoic acid.
Overall Reaction
- The overall reaction can be summarized as follows:
- Bromobenzene + KCN → Benzonitrile
- Benzonitrile + H2O/HCl → Benzoic Acid
Advantages of Using KCN
- One-pot reaction: The process is straightforward and can be achieved in two steps.
- Higher yields: The method might provide higher yields compared to other methods.
- Functional group tolerance: This method is versatile and can be adapted for similar aromatic compounds.
Conclusion
Using KCN followed by hydrolysis is a viable alternative method for converting bromobenzene to benzoic acid. This approach not only simplifies the reaction but also enhances the efficiency of the transformation.
Bromobenzene can indeed be converted to benzoic acid using potassium cyanide (KCN) followed by hydrolysis. Here’s how the process works:
Step 1: Nucleophilic Substitution
- Bromobenzene contains a bromine atom, which is a good leaving group.
- When treated with KCN, a nucleophilic substitution reaction occurs.
- The cyanide ion (CN-) acts as a nucleophile, attacking the carbon atom bonded to bromine.
- This results in the formation of phenyl cyanide (benzonitrile).
Step 2: Hydrolysis
- The next step involves hydrolysis of the benzonitrile.
- Hydrolysis can be performed using aqueous acid (HCl) or a base (NaOH) under heat.
- In this reaction, the nitrile group (−C≡N) is converted to a carboxylic acid group (−COOH).
- This results in the formation of benzoic acid.
Overall Reaction
- The overall reaction can be summarized as follows:
- Bromobenzene + KCN → Benzonitrile
- Benzonitrile + H2O/HCl → Benzoic Acid
Advantages of Using KCN
- One-pot reaction: The process is straightforward and can be achieved in two steps.
- Higher yields: The method might provide higher yields compared to other methods.
- Functional group tolerance: This method is versatile and can be adapted for similar aromatic compounds.
Conclusion
Using KCN followed by hydrolysis is a viable alternative method for converting bromobenzene to benzoic acid. This approach not only simplifies the reaction but also enhances the efficiency of the transformation.
Community Answer
For conversion of bromobenzene to benzoic acid the given method is giv...

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead?
Question Description
For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead?.
For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead?.
Solutions for For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead? defined & explained in the simplest way possible. Besides giving the explanation of
For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead?, a detailed solution for For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead? has been provided alongside types of For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead? theory, EduRev gives you an
ample number of questions to practice For conversion of bromobenzene to benzoic acid the given method is given in all books. Can KCN be used followed by hydrolysis instead? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















