Physics Exam > Physics Questions > Yellow light of 557nmwavelength is incident o...
Start Learning for Free
Yellow light of 557 nm wavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (in nm) for photoelectric effect from cesium is.
Correct answer is '625'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Yellow light of 557nmwavelength is incident on a cesium surface. It is...
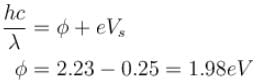
⇒
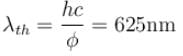
The correct answer is: 625
Most Upvoted Answer
Yellow light of 557nmwavelength is incident on a cesium surface. It is...
Explanation:
To understand the threshold wavelength for the photoelectric effect from cesium, we need to consider the relationship between the energy of a photon and the work function of the material.
1. Energy of a Photon:
The energy of a photon can be calculated using the equation:
E = hc/λ
where E is the energy of the photon, h is the Planck's constant (6.63 x 10^-34 J.s), c is the speed of light (3 x 10^8 m/s), and λ is the wavelength of the light.
2. Work Function:
The work function is the minimum amount of energy required to remove an electron from the surface of a material. It can be represented as:
φ = hc/λ0
where φ is the work function and λ0 is the threshold wavelength.
3. Threshold Wavelength:
The threshold wavelength is the wavelength of the light below which the photoelectric effect does not occur. It can be determined by rearranging the equation for the work function:
λ0 = hc/φ
4. Calculation:
In this case, we are given that the yellow light incident on the cesium surface has a wavelength of 557 nm. We are also given that the cathode-anode voltage drops below 0.25 V when no photo-electron flow occurs.
To find the threshold wavelength, we can rearrange the equation for the work function:
λ0 = hc/φ
Since the cathode-anode voltage drops below 0.25 V, the maximum kinetic energy of the emitted electrons is given by:
KEmax = eV
where e is the elementary charge (1.6 x 10^-19 C) and V is the voltage.
The energy of a photon can be related to the maximum kinetic energy of an electron using the equation:
E = KEmax + φ
Substituting the values and rearranging the equation, we get:
hc/λ = eV + φ
Since no photo-electron flow occurs when the cathode-anode voltage drops below 0.25 V, the maximum kinetic energy of the emitted electrons is zero. Therefore:
0 = e(0.25) + φ
Simplifying the equation, we find:
φ = -0.25e
Now we can substitute this value into the equation for the threshold wavelength:
λ0 = hc/φ
λ0 = hc/(-0.25e)
Calculating the value, we find:
λ0 ≈ 625 nm
Therefore, the threshold wavelength for the photoelectric effect from cesium is approximately 625 nm.
To understand the threshold wavelength for the photoelectric effect from cesium, we need to consider the relationship between the energy of a photon and the work function of the material.
1. Energy of a Photon:
The energy of a photon can be calculated using the equation:
E = hc/λ
where E is the energy of the photon, h is the Planck's constant (6.63 x 10^-34 J.s), c is the speed of light (3 x 10^8 m/s), and λ is the wavelength of the light.
2. Work Function:
The work function is the minimum amount of energy required to remove an electron from the surface of a material. It can be represented as:
φ = hc/λ0
where φ is the work function and λ0 is the threshold wavelength.
3. Threshold Wavelength:
The threshold wavelength is the wavelength of the light below which the photoelectric effect does not occur. It can be determined by rearranging the equation for the work function:
λ0 = hc/φ
4. Calculation:
In this case, we are given that the yellow light incident on the cesium surface has a wavelength of 557 nm. We are also given that the cathode-anode voltage drops below 0.25 V when no photo-electron flow occurs.
To find the threshold wavelength, we can rearrange the equation for the work function:
λ0 = hc/φ
Since the cathode-anode voltage drops below 0.25 V, the maximum kinetic energy of the emitted electrons is given by:
KEmax = eV
where e is the elementary charge (1.6 x 10^-19 C) and V is the voltage.
The energy of a photon can be related to the maximum kinetic energy of an electron using the equation:
E = KEmax + φ
Substituting the values and rearranging the equation, we get:
hc/λ = eV + φ
Since no photo-electron flow occurs when the cathode-anode voltage drops below 0.25 V, the maximum kinetic energy of the emitted electrons is zero. Therefore:
0 = e(0.25) + φ
Simplifying the equation, we find:
φ = -0.25e
Now we can substitute this value into the equation for the threshold wavelength:
λ0 = hc/φ
λ0 = hc/(-0.25e)
Calculating the value, we find:
λ0 ≈ 625 nm
Therefore, the threshold wavelength for the photoelectric effect from cesium is approximately 625 nm.
Free Test
FREE
| Start Free Test |
Community Answer
Yellow light of 557nmwavelength is incident on a cesium surface. It is...
What is frequency of incident photon

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer?
Question Description
Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer?.
Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer?.
Solutions for Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer?, a detailed solution for Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer? has been provided alongside types of Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Yellow light of 557nmwavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (innm) for photoelectric effect from cesium is.Correct answer is '625'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















